DNA 纳米贴片特异性改造益生菌,用于超声诱发的炎症性肠病治疗
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
益生菌为治疗炎症性肠病提供了可喜的成果,但精准治疗仍具有挑战性,尤其是在空间和时间上操纵益生菌并使其免受氧化应激方面。为了解决这些局限性,我们在本文中合成了细菌特异性 DNA 纳米贴片,以修饰超声触发的工程化大肠埃希氏菌 Nissle 1917。这些益生菌在受到超声波刺激时会产生抗炎细胞因子白细胞介素-10,并通过强化 DNPs 来抵抗氧化应激。DNPs 由具有活性氧清除能力的矩形 DNA 折纸纳米片和麦芽糊精分子的细菌靶向配体组成。我们系统地证明了 DNPs 可通过麦芽糊精转运途径选择性地附着在细菌表面,而不能附着在哺乳动物细胞表面。为了进一步提高工程益生菌在胃肠道中的生物利用率,我们采用了一种自组装策略,利用壳聚糖和海藻酸钠将它们包裹起来。在溃疡性结肠炎的小鼠模型中,该系统显著改善了肠道屏障的完整性并减轻了炎症。我们的研究结果表明,这种 DNA 纳米贴片-细菌系统在减轻氧化应激、纠正微生物群失调、增强结肠炎肠道屏障功能方面大有可为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
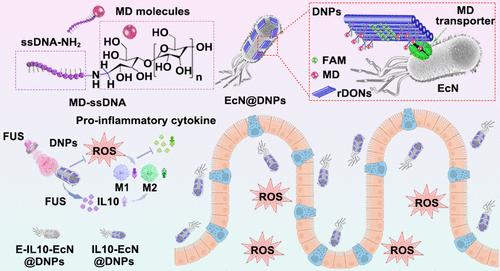
DNA Nanopatch-Specific Modification of Probiotics for Ultrasound-Triggered Inflammatory Bowel Disease Therapy
Probiotics offer promising results for treating inflammatory bowel disease, yet precision therapy remains challenging, particularly in manipulating probiotics spatially and temporally and shielding them from oxidative stress. To address these limitations, herein we synthesized bacteria-specific DNA nanopatches to modify ultrasound-triggered engineered Escherichia coli Nissle 1917. These probiotics produced the anti-inflammatory cytokine interleukin-10 when stimulated by ultrasound and were fortified with DNPs for oxidative stress resistance. The DNPs were composed of rectangular DNA origami nanosheets with reactive oxygen species’ scavenging ability and bacterial targeting ligands of maltodextrin molecules. We systematically demonstrated that the DNPs could selectively attach to bacterial surface but not mammalian cell surface via the maltodextrin transporter pathway. To further enhance the bioavailability of engineered probiotics in the gastrointestinal tract, we employed a self-assembly strategy to encapsulate them using chitosan and sodium alginate. In a murine model of ulcerative colitis, this system significantly improved the gut barrier integrity and reduced inflammation. Our results indicate that this DNA nanopatch-bacteria system holds substantial promise for mitigating oxidative stress, correcting microbiota dysbiosis, and enhancing the intestinal barrier function in colitis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


