微量水分导致阴极合成过程中的锂挥发
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
锂离子电池正极的合成通常是在高温下通过混合金属氢氧化物沉淀与锂源之间的反应进行的。虽然传闻证据表明,部分锂可能会在热处理过程中蒸发,但此前尚未发表过关于锂挥发性的热力学分析。锂蒸气的形成有利于克服固-固反应中的质量传输限制,但也可能导致锂的损失,并改变最终产品的预定化学计量。为了严格量化锂汽化在热力学上的可行程度以及所涉及的锂物种的性质,我们对气态分子锂氧化物和氢氧化物进行了高水平量子化学计算,并根据吉布斯自由能考虑因素评估了它们的形成范围。结果表明,虽然氧化锂在干燥条件下的挥发性可以忽略不计,但ppm 浓度的水蒸气足以通过气态 LiOH 中间体显著提高锂的汽化。因此,在合成过程中控制湿度对生产高质量的锂离子正极材料至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
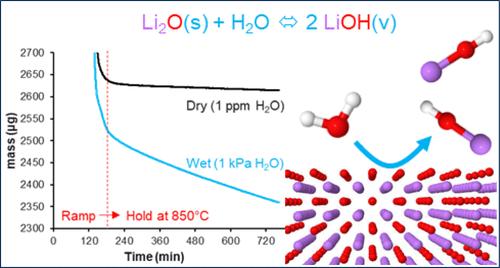
Trace Moisture Drives Lithium Volatility during Cathode Synthesis
Synthesis of cathodes for lithium ion batteries is typically conducted via a reaction between a mixed metal hydroxide precipitate and a lithium source at elevated temperatures. While anecdotal evidence suggests that some portion of lithium may vaporize during thermal treatment, a thermodynamic analysis of lithium volatility has not been previously published. The formation of lithium vapor would be beneficial for overcoming mass transport limitations in otherwise solid–solid reactions but may also result in lithium loss and alteration of the intended stoichiometry of the final product. To rigorously quantify the extent to which lithium vaporization is thermodynamically feasible and the nature of the lithium species involved, high-level quantum chemical calculations have been performed on gaseous molecular lithium oxides and hydroxides and their extents of formation assessed from Gibbs Free Energy considerations. Results show that while the volatility of lithium oxide is negligible under dry conditions, ppm concentrations of water vapor are sufficient to dramatically enhance lithium vaporization via gaseous LiOH intermediates. Controlling the moisture level during synthesis is therefore of paramount importance in the production of high-quality lithium ion cathode materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


