氧化铍(Be12O12)和氮化硼(B12N12)纳米囊作为别嘌醇药物强效给药系统的 DFT 比较研究
IF 2.8
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用 DFT 方法研究了 Be12O12 和 B12N12 纳米笼作为别嘌呤醇(APN)给药系统的潜力。APN∙∙Be12O12 和 ∙∙B12N12 复合物的大量负相互作用和吸附能证实了吸附过程。SAPT分析表明,静电力在相互作用中占主导地位。对 APN∙∙∙nanocage 复合物中分子间的相互作用进行了深入研究。对 TDOS 和 PDOS 的分析表明,APN 在 Be12O12 和 B12N12 中的负载量很大。在 APN∙∙MgBe11O12 和 ∙∙AlB11N12 复合物中,也分别估计了掺杂原子对 APN 吸附过程的实质性影响。APN 负载过程的性质被证实是自发和放热的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
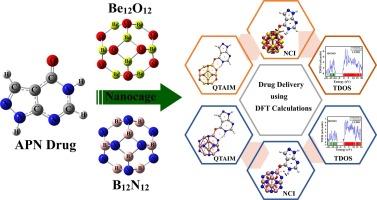
A comparative DFT study of beryllium oxide (Be12O12) and boron nitride (B12N12) nanocages as potent drug delivery systems for allopurinol drug
The potential of Be12O12 and B12N12 nanocages as Allopurinol (APN) drug delivery systems was investigated using DFT methods. The adsorption process was confirmed by substantial negative interaction and adsorption energies of APN∙∙∙Be12O12 and ∙∙∙B12N12 complexes. SAPT analysis indicated that electrostatic forces significantly dominated the interactions. Intermolecular interactions within APN∙∙∙nanocage complexes were thoroughly characterized. The analysis of TDOS and PDOS assured substantial loading of APN over Be12O12 and B12N12. The substantial effect of doped atoms on the APN adsorption process was also estimated in APN∙∙∙MgBe11O12 and ∙∙∙AlB11N12 complexes, respectively. The nature of the APN-loading process was affirmed to be spontaneous and exothermic.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


