使用氯化胆碱盐和水基深共晶溶剂的乙腈-水体系液-液相平衡行为比较
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在298 K的温度下测量了乙腈(ACN)+水(W)+氯化胆碱(ChCl)和ACN+W+由ChCl:W(1:3)组成的水基深共晶溶剂(WDES)三元体系的液液平衡(LLE)数据,并通过Bachman和Othmer-Tobias相关性验证了平衡线的一致性。与其他无机盐和疏水性DES相比,作为有机盐的ChCl及其WDES(含水率范围小于50 w%)作为绿色萃取剂,在从乙腈-水体系中分离水的选择性和分配系数方面表现出可行的性能。ACN + W + ChCl)体系中的 ChCl 能以水合物离子的形式将水从溶液中分离出来,而(ACN + W + WDES)体系中的 WDES 则倾向于通过分子间作用力吸水。使用对称电解质非随机双液(e-NRTL)模型对(ACN + W + ChCl)体系的实验数据进行了关联,而使用 NRTL 和通用准化学活度系数(UNIQUAC)模型对(ACN + W + WDES)体系的平衡数据进行了关联。在从乙腈混合物中去除水分方面,盐形式的 ChCl 比 WDES 具有更好的分离性能。同时,就再生能耗和操作考虑而言,WDES 可被视为优于 ChCl。本文章由计算机程序翻译,如有差异,请以英文原文为准。
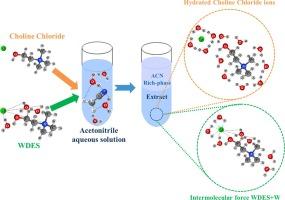
Comparison of liquid–liquid phase equilibrium behavior of acetonitrile–water system using choline chloride salt and water-based deep eutectic solvent
The liquid–liquid equilibrium (LLE) data for the ternary systems of (acetonitrile (ACN) + water (W) + choline chloride (ChCl)) and (ACN + W + water-based deep eutectic solvent (WDES) consisting of ChCl:W (1:3)) were measured at 298 K. The consistency of tie-lines was verified by the Bachman and Othmer–Tobias correlations. ChCl as an organic salt and its WDES (within the water content range of less than 50 w%) as green extractants represent feasible performances in terms of selectivity and distribution coefficients for separation of water from acetonitrile-water system in comparison to other inorganic salts and hydrophobic DESs. ChCl in the (ACN + W + ChCl) system can separate water from the solution in the form of hydrate ions while WDES in the (ACN + W + WDES) system tends to absorb water by intermolecular forces. The experimental data of the (ACN + W + ChCl) system was correlated using the symmetric electrolyte non-random two liquid (e-NRTL) model while the equilibrium data of the (ACN + W + WDES) system was correlated using NRTL and universal quasi-chemical activity coefficient (UNIQUAC) models. ChCl in the form of salt represents a better separation performance than WDES for water removal from the acetonitrile mixture. Meanwhile, WDES might be considered to be superior compared to ChCl in terms of regeneration energy consumption and operating considerations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


