离子液体 (IL) 在氮化石墨碳 (g-C3N4) 纳米片上的界面吸附:DFT 研究
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用密度泛函理论(DFT)方法在气相和水溶剂中研究了九种常见离子液体(ILs)在两种著名的氮化石墨碳(g-C3N4)纳米片(包括三嗪-g-C3N4和七嗪-g-C3N4)上的吸附。通过分子中原子量子理论(QTAIM)分析、非共价相互作用(NCI)图和能量分解分析(EDA)法等几种技术对 IL 与纳米片之间的相互作用进行了表征。有趣的是,所得结果显示,静电相互作用对 IL 与表面之间的相互作用能贡献最大,其次是分散相互作用和轨道相互作用(ΔEelec > ΔEdisp > ΔEorb)。IL 与表面之间最重要的范德华(vdW)相互作用包括π-π、CH...N、CH...π(CN)和 CN...X(X = F、N、O)相互作用。另一方面,IL 的吸附伴随着从表面到 IL 的电荷转移。计算得出的吸附能(Eads)表明,[Bmim][PF6] IL 在三嗪-g-C3N4(-28.28 kcal/mol)和七嗪-g-C3N4(-26.68 kcal/mol)表面的吸附亲和力最高。热化学计算表明,IL@表面复合物的Eads、ΔHads和ΔGads值在从气相转移到水介质时会降低。然而,我们的研究结果表明,IL 在表面的吸附仍然是放热的,并且是自发进行的。吸附 IL 后,观察到 HOMO-LUMO 能隙 (Eg) 减小,同时表面的反应性和亲电性增强。TD-DFT 计算表明,IL 的微弱吸附会导致三嗪-g-C3N4 和七嗪-g-C3N4 表面的吸收带发生微小的红移或蓝移。然而,在表面吸收光谱的形状上没有观察到明显的变化。此外,IL@表面复合物的跃迁密度矩阵(TDM)热图显示,电子和空穴一般集中在IL@表面复合物中的三嗪-g-C3N4和庚嗪-g-C3N4表面,这表明电子跃迁主要发生在表面上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
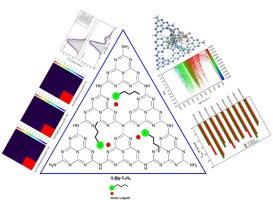
Interfacial adsorption of ionic liquids (ILs) on graphitic carbon nitride (g-C3N4) nanosheets: A DFT study
The adsorption of nine common ionic liquids (ILs) on two well-known structures of graphitic carbon nitride (g-C3N4) nanosheets, including Triazine-g-C3N4 and Heptazine-g-C3N4 has been investigated using density functional theory (DFT) method in gas phase and water solvent. The interactions between the ILs and the nanosheets are characterized by several techniques, such as quantum theory of atoms in molecules (QTAIM) analysis, noncovalent interaction (NCI) plots, and energy decomposition analysis (EDA) method. Interestingly, the obtained results showed that electrostatic interactions have the highest contributions to the interaction energies between the ILs and the surfaces, followed by dispersion interactions and orbital interactions (ΔEelec > ΔEdisp > ΔEorb) The most important Van der Waals (vdW) interactions between the ILs and the surfaces include π–π, C![]() H…N, C
H…N, C![]() H…π (C
H…π (C![]() N), and C
N), and C![]() N…X (X = F, N, O) interactions. On the other hand, the adsorption of ILs is accompanied by charge transfer from the surfaces to the ILs. The calculated adsorption energy (Eads) showed that the [Bmim][PF6] IL has the highest adsorption affinity on the Triazine-g-C3N4 (−28.28 kcal/mol) and Heptazine-g-C3N4 (−26.68 kcal/mol) surfaces. Thermochemistry calculations showed that the Eads, ΔHads, and ΔGads values of IL@surface complexes decrease on moving from gas phase to water media. However, our results showed that the adsorption of ILs on the surfaces is still exothermic and proceeds spontaneously. Upon adsorption of ILs, a decrease in the HOMO–LUMO energy gap (Eg) is observed and is accompanied by an increase in the reactivity and electrophilic character of the surfaces. TD-DFT calculations showed that the weak adsorption of ILs leads to small red or blue shifts in the absorption bands of the Triazine-g-C3N4 and Heptazine-g-C3N4 surfaces. However, no significant changes are observed in the shape of the absorption spectra of the surfaces. Furthermore, the transition density matrix (TDM) heat maps of the IL@surface complexes showed that the electrons and holes are generally concentrated on the Triazine-g-C3N4 and Heptazine-g-C3N4 surfaces in the IL@surface complexes, signifying that the electronic transitions occur mainly on the surfaces.
N…X (X = F, N, O) interactions. On the other hand, the adsorption of ILs is accompanied by charge transfer from the surfaces to the ILs. The calculated adsorption energy (Eads) showed that the [Bmim][PF6] IL has the highest adsorption affinity on the Triazine-g-C3N4 (−28.28 kcal/mol) and Heptazine-g-C3N4 (−26.68 kcal/mol) surfaces. Thermochemistry calculations showed that the Eads, ΔHads, and ΔGads values of IL@surface complexes decrease on moving from gas phase to water media. However, our results showed that the adsorption of ILs on the surfaces is still exothermic and proceeds spontaneously. Upon adsorption of ILs, a decrease in the HOMO–LUMO energy gap (Eg) is observed and is accompanied by an increase in the reactivity and electrophilic character of the surfaces. TD-DFT calculations showed that the weak adsorption of ILs leads to small red or blue shifts in the absorption bands of the Triazine-g-C3N4 and Heptazine-g-C3N4 surfaces. However, no significant changes are observed in the shape of the absorption spectra of the surfaces. Furthermore, the transition density matrix (TDM) heat maps of the IL@surface complexes showed that the electrons and holes are generally concentrated on the Triazine-g-C3N4 and Heptazine-g-C3N4 surfaces in the IL@surface complexes, signifying that the electronic transitions occur mainly on the surfaces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


