合成具有体外抗胰腺癌细胞增殖活性的α-氟肉桂酸酯衍生物作为新型胰蛋白酶 S 抑制剂
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
胰蛋白酶是半胱氨酸蛋白酶木瓜蛋白酶样家族的重要成员,对人体细胞内的蛋白分解过程至关重要,包括骨溶解、免疫调节和细胞凋亡。最近的研究突显了胰蛋白酶,尤其是 L、S、K 和 B 亚型胰蛋白酶在胰腺癌中的重要作用。这推动了新型胰蛋白酶抑制剂的开发,作为抑制肿瘤进展、迁移和侵袭的潜在治疗手段。在临床前模型中,以猫嗜蛋白酶 S(CatS)为靶点有望减少肿瘤进展并提高化疗药物的疗效。我们以前曾利用对氨基肉桂酸乙酯衍生物对半胱氨酸蛋白酶进行共价抑制,在此基础上,我们设计并合成了三种新的衍生物,它们基于肉桂酸分子水平上的同位取代(H-F)。这些衍生物是 CatS 的强效共价抑制剂(1.8-2.6 µM),其中 2F 对 CatL(20%)和 CatB(29%)也有微弱的抑制活性。2F 对胰腺癌细胞株 BXPC3 和 CAPAN1 的体外检测显示出显著的抗增殖活性,IC50 分别为 5.79 µM 和 20.75 µM。这些发现凸显了α-氟肉桂酸半胱氨酸蛋白酶抑制剂的潜力,有望进一步开发用于靶向CatS和CatL,以减少胰腺癌细胞增殖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
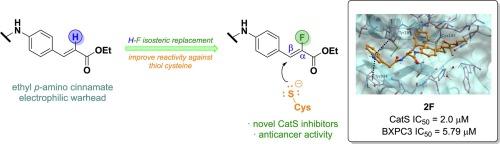
Synthesis of α-fluorocinnamate derivatives as novel cathepsin S inhibitors with in vitro antiproliferative activity against pancreatic cancer cells
Cathepsins, key members of the papain-like family of cysteine proteases, are crucial for proteolysis processes within human cells, including osteolysis, immunomodulation and apoptosis. Recent research has highlighted the significant role of cathepsins, particularly the L, S, K, and B subtypes, in pancreatic cancer. This has driven the development of novel cathepsin inhibitors as potential treatments to inhibit tumor progression, migration and invasion. Targeting cathepsin S (CatS) has shown promise in reducing tumor progression and enhancing the efficacy of chemotherapeutic agents in preclinical models. Building on our previous work where we employed ethyl p-aminocinnamate ester derivatives for covalent inhibition of cysteine proteases, herein we have designed and synthesized three new derivatives basing on an isosteric replacement (H–F) at the level of cinnamate moiety. These derivatives emerged as potent covalent inhibitors of CatS (1.8–2.6 µM) with 2F showing also weak inhibition activity against CatL (20 %) and CatB (29 %). In vitro assays of 2F against pancreatic cancer cell lines BXPC3 and CAPAN1 revealed significant antiproliferative activity, with IC50 = 5.79 µM and 20.75 µM, respectively. These findings underscore the potential of α-fluorocinnamate-based cysteine protease inhibitors as promising candidates for further development in targeting CatS and CatL with the aim to reduce pancreatic cancer cell proliferation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


