高级联合疗法对免疫介导的炎症性疾病的疗效和安全性:随机对照试验的系统回顾和荟萃分析
IF 7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
目的 先进的联合治疗(ACT)是指至少两种生物制剂、一种生物制剂和一种口服小分子药物、两种具有不同作用机制的口服小分子药物的联合治疗,是改善免疫介导的炎症性疾病(IMID)患者预后的一种拟议策略。我们对随机对照试验(RCT)进行了系统综述和荟萃分析,比较了ACT与单药疗法对特定IMID患者的治疗效果。方法通过系统文献检索,我们发现了10项比较ACT与单药疗法(单药疗法)的RCT(n = 1154)。主要结果是诱导临床缓解。次要结果为不良事件、严重不良事件、感染和严重感染。结果10项试验中有8项研究了抗TNF-α药物(如依那西普、英夫利昔单抗、戈利木单抗、certolizumab)与另一种生物制剂(如抗IL-23、抗整合素、抗IL-1)或口服小分子药物的联合治疗。在类风湿性关节炎患者(n = 7 项 RCTs)(RR,1.75 [95 % CI 0.60-5.13];中度异质性(I2 = 33 %))和系统性红斑狼疮患者(n = 1 项)(RR,1.20 [0.53-2.72])中,ACT 与单药治疗实现临床缓解的可能性无明显差异(GRADE;低确定性证据)。与单一疗法相比,ACT治疗组的类风湿关节炎患者更容易出现不良反应(RR,1.07 [1.01-1.12])。ACT 可使 IBD 患者的临床缓解率提高(n = 2 项 RCTs)(RR,1.68 [1.15-2.46]),异质性极小(I2 = 15%)(GRADE;证据确定性低),不良事件发生的可能性无差异(RR 0.92 [0.80-1.05])。在接受 ACT 或单药治疗的风湿病或 IBD 患者中,感染或严重感染的风险没有差异。结论ACT 对风湿病 IMIDs 患者没有临床益处,对类风湿性关节炎患者的不良事件发生率较高。ACT可能会给IBD患者带来临床益处,但没有明确的安全性信号,因此有必要进行进一步的试验。不同研究中的 ACT 方案存在差异,这限制了这些研究结果的推广性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
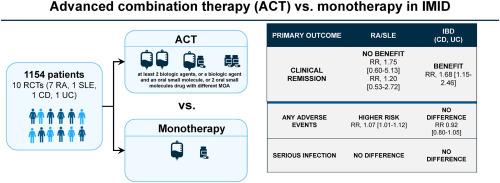
Efficacy and safety of Advanced Combination Treatment in immune-mediated inflammatory disease: A systematic review and meta-analysis of randomized controlled trials
Objectives
Advanced combination treatment (ACT), defined as a combination of at least 2 biologic agents, a biologic agent and an oral small molecule, 2 oral small molecules drug with different mechanisms of action is a proposed strategy to improve outcomes in patients with immune-mediated inflammatory disease (IMID). We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing ACT with monotherapy in patients with select IMIDs.
Methods
Through a systematic literature search, we identified 10 RCTs (n = 1154) comparing ACT with single agent therapy (monotherapy). The primary outcome was induction of clinical remission. Secondary outcomes were adverse events, serious adverse events, infections, and serious infections. We performed random-effects meta-analysis and used GRADE to appraise certainty of evidence.
Results
Eight out of 10 trials investigated an anti-TNF-α drug (e.g., etanercept, infliximab, golimumab, certolizumab) combined with another biologic (e.g anti-IL-23, anti-integrin, anti-IL-1) or an oral small molecule. There was no significant difference in the likelihood of achieving clinical remission with ACT vs. monotherapy in patients with rheumatoid arthritis (n = 7 RCTs) (RR, 1.75 [95 % CI 0.60–5.13]; moderate heterogeneity (I2 = 33 %)] and systemic lupus erythematosus (n = 1) (RR, 1.20 [0.53–2.72]) (GRADE; low certainty evidence). Patients with rheumatoid arthritis in the ACT arm were more likely to experience adverse events (RR, 1.07 [1.01–1.12]) compared to monotherapy. ACT led to higher rates of induction of clinical remission in patients with IBD (n = 2 RCTs) (RR, 1.68 [1.15–2.46]) with minimal heterogeneity (I2 = 15 %) (GRADE; low certainty evidence), and no differences in the likelihood of adverse events (RR 0.92 [0.80–1.05]). There were no differences in the risk of infections or serious infections in patients treated with ACT or monotherapy with rheumatological disease or IBD.
Conclusions
ACT did not offer clinical benefit in patients with rheumatological IMIDs and resulted in higher rate adverse events in rheumatoid arthritis. ACT may offer clinical benefit without a clear safety signal in patients with IBD, but further trials are warranted. The variability in ACT regimens across studies limits the generalizability of these findings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of autoimmunity
医学-免疫学
CiteScore
27.90
自引率
1.60%
发文量
117
审稿时长
17 days
期刊介绍:
The Journal of Autoimmunity serves as the primary publication for research on various facets of autoimmunity. These include topics such as the mechanism of self-recognition, regulation of autoimmune responses, experimental autoimmune diseases, diagnostic tests for autoantibodies, as well as the epidemiology, pathophysiology, and treatment of autoimmune diseases. While the journal covers a wide range of subjects, it emphasizes papers exploring the genetic, molecular biology, and cellular aspects of the field.
The Journal of Translational Autoimmunity, on the other hand, is a subsidiary journal of the Journal of Autoimmunity. It focuses specifically on translating scientific discoveries in autoimmunity into clinical applications and practical solutions. By highlighting research that bridges the gap between basic science and clinical practice, the Journal of Translational Autoimmunity aims to advance the understanding and treatment of autoimmune diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


