果蝇 RNA 结合蛋白 Hrp48 与 msl-2 mRNA 3' UTR 的特定 RNA 序列结合,从而调节翻译。
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
抑制msl-2 mRNA的翻译对黑腹果蝇雌体的存活至关重要,以防止两条X染色体的过度转录。这种翻译控制事件由雌性特异性蛋白Sex-lethal(Sxl)协调,Sxl将RNA结合蛋白Unr和Hrp48招募到msl-2转录本的3'非翻译区(UTR),并抑制翻译启动。Hrp48在翻译抑制过程中的作用机制及其与msl-2的相互作用尚不十分清楚。在这里,我们研究了 Hrp48 的串联 RNA 识别基团的 RNA 结合特异性和亲和性。利用核磁共振光谱、分子动力学模拟和等温滴定量热法,我们确定了 Hrp48 识别的 msl-2 3' UTR 的确切区域。其他生物物理实验和翻译测定进一步揭示了 Hrp48、Unr、Sxl 和 RNA 形成复合物的情况。我们的结果表明,Hrp48与msl-2的Sxl和Unr的E和F结合位点下游结合,而不依赖于Sxl和Unr。本文章由计算机程序翻译,如有差异,请以英文原文为准。
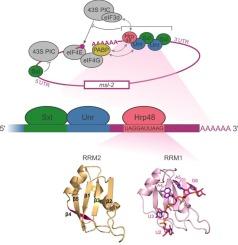
The Drosophila RNA binding protein Hrp48 binds a specific RNA sequence of the msl-2 mRNA 3’ UTR to regulate translation
Repression of msl-2 mRNA translation is essential for viability of Drosophila melanogaster females to prevent hypertranscription of both X chromosomes. This translational control event is coordinated by the female-specific protein Sex-lethal (Sxl) which recruits the RNA binding proteins Unr and Hrp48 to the 3’ untranslated region (UTR) of the msl-2 transcript and represses translation initiation. The mechanism exerted by Hrp48 during translation repression and its interaction with msl-2 are not well understood. Here we investigate the RNA binding specificity and affinity of the tandem RNA recognition motifs of Hrp48. Using NMR spectroscopy, molecular dynamics simulations and isothermal titration calorimetry, we identified the exact region of msl-2 3’ UTR recognized by Hrp48. Additional biophysical experiments and translation assays give further insights into complex formation of Hrp48, Unr, Sxl and RNA. Our results show that Hrp48 binds independent of Sxl and Unr downstream of the E and F binding sites of Sxl and Unr to msl-2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


