2-Arylhydrazinylidene-3-oxo-3-polyfluoroalkylpropanoic acids 作为具有强大抗氧化潜力的选择性有效羧酯酶抑制剂。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
通过 AlBr3 对烷基-2-芳基肼亚基-3-氧代-3-烷酸进行脱烷基反应,制备了一系列 2-芳基肼亚基-3-氧代酸(AHOAs)。利用 X 射线、核磁共振光谱和量子力学计算(QM),确定了 AHOAs 存在于热力学上有利的 Z 形式中,并由两个分子内 H 键稳定。所有 AHOAs 的 ADME 参数均可接受。酯酶谱研究表明,多氟烷基 AHOAs 是有效的选择性羧基酯酶(CES)抑制剂,而对乙酰胆碱酯酶和丁酰胆碱酯酶无活性。与分子对接结果一致,最有效的 CES 抑制剂(IC50 低至 42 nM)是含有长多氟烷基取代基的化合物。这些酸类对 hCES1 和 hCES2 也有活性,含 CF3 的酸类对 hCES2 具有选择性。非氟化酸对 CES 没有抑制作用,但它们具有很强的抗氧化能力。在 ABTS、FRAP 和 ORAC 试验中,芳基肼亚基中含有未取代的苯基或电子奉献基团的 AHOAs 显示出较高的一级抗氧化活性,而在 ABTS 和 ORAC 试验中,这种活性与酰基片段中的取代基无关。利用 QM 计算研究了 AHOAs 的自由基清除机理,结果表明它们更倾向于裂解 NH 键而不是 OH 键。结果表明,4-甲氧基取代的 AHOAs 在 AAPH 诱导的氧化应激条件下对红细胞膜具有保护作用,包括膜稳定活性、抑制 AAPH 诱导的红细胞膜脂质过氧化反应和铁(II)螯合能力。因此,我们开发出了一类具有强大抗氧化潜力的新型强效选择性 CES 抑制剂,它们是很有前景的辅助药物,能够调节酯化药物的代谢,清除第一阶段生物转化过程中形成的活性自由基。本文章由计算机程序翻译,如有差异,请以英文原文为准。
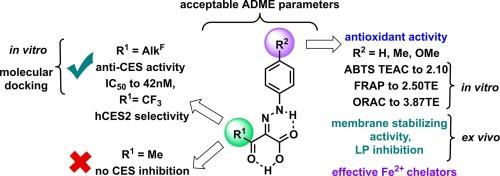
2-Arylhydrazinylidene-3-oxo-3-polyfluoroalkylpropanoic acids as selective and effective carboxylesterase inhibitors with powerful antioxidant potential
A series of 2-arylhydrazinylidene-3-oxo acids (AHOAs) was prepared by dealkylation of alkyl-2-arylhydrazinylidene-3-oxo-3-alkanoates with AlBr3. Using X-Ray, NMR spectroscopy, and quantum mechanical calculations (QM), the existence of AHOAs in a thermodynamically favorable Z-form stabilized by two intramolecular H-bonds was established. All AHOAs had acceptable ADME parameters. The esterase profile study showed that polyfluoroalkyl-AHOAs were effective and selective carboxylesterase (CES) inhibitors, while they were inactive against acetyl- and butyrylcholinesterase. In agreement with molecular docking, the most effective CES inhibitors (IC50 as low as 42 nM) were compounds bearing long polyfluoroalkyl substituents. The acids were also active against hCES1 and hCES2, and CF3-containing acids possessed selectivity against hCES2. Non-fluorinated acids did not inhibit CES, but they exhibited potent antioxidant capability. AHOAs having unsubstituted phenyl or electron-donating groups in the arylhydrazinylidene moiety displayed high primary antioxidant activity in the ABTS, FRAP, and ORAC tests, which did not depend on the substituent in the acyl fragment in the ABTS and ORAC assays. The radical-scavenging mechanism of AHOAs was investigated using QM calculations, showing a preference for cleavage of N![]() H rather than O
H rather than O![]() H bonds. For the lead antioxidants, 4-methoxysubstituted AHOAs, protective effects on erythrocyte membranes in AAPH-induced oxidative stress conditions were shown, including membrane stabilizing activity, inhibition of AAPH-induced lipid peroxidation of erythrocyte membranes, and Fe(II)-chelating ability. Thus, a new class of potent and selective CES inhibitors with powerful antioxidant potential has been developed as promising co-drugs capable of regulating the metabolism of esterified drugs and scavenging reactive radicals that form during Phase I biotransformation.
H bonds. For the lead antioxidants, 4-methoxysubstituted AHOAs, protective effects on erythrocyte membranes in AAPH-induced oxidative stress conditions were shown, including membrane stabilizing activity, inhibition of AAPH-induced lipid peroxidation of erythrocyte membranes, and Fe(II)-chelating ability. Thus, a new class of potent and selective CES inhibitors with powerful antioxidant potential has been developed as promising co-drugs capable of regulating the metabolism of esterified drugs and scavenging reactive radicals that form during Phase I biotransformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


