SMARCAL1 和 FANCM 之间的合成致死率很高
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
DNA 复制压力是对基因组完整性的一种威胁。大型 SNF2 ATP 酶家族利用 ATP 驱动的马达重塑 DNA 或 DNA 结合蛋白,从而参与预防和减轻 DNA 复制压力。为了了解这些 ATP 酶在基因组维护中的贡献,我们在人体细胞中利用三种 SNF2 型 ATP 酶进行了基于 CRISPR 的合成致死筛选:SMARCAL1、ZRANB3 和 HLTF。在这里,我们发现 SMARCAL1 与 FANCM(另一种参与 DNA 复制和基因组稳定性的 ATP 依赖性转运酶)之间存在深远的合成致死相互作用。它们的共同缺失会导致严重的基因组不稳定性,我们将这种不稳定性与染色体在富含简单重复序列的位点上的断裂联系起来,众所周知,简单重复序列会挑战复制叉的进展。我们的发现揭示了一种关键的遗传缓冲机制,它为维持基因组完整性提供了重要功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
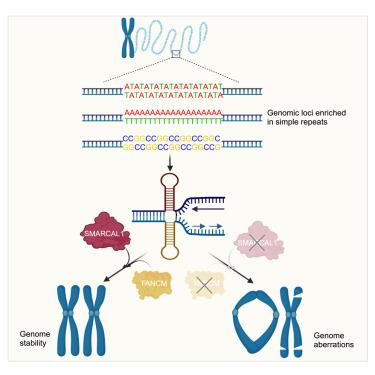
Profound synthetic lethality between SMARCAL1 and FANCM
DNA replication stress is a threat to genome integrity. The large SNF2-family of ATPases participates in preventing and mitigating DNA replication stress by employing their ATP-driven motor to remodel DNA or DNA-bound proteins. To understand the contribution of these ATPases in genome maintenance, we undertook CRISPR-based synthetic lethality screens in human cells with three SNF2-type ATPases: SMARCAL1, ZRANB3, and HLTF. Here, we show that SMARCAL1 displays a profound synthetic-lethal interaction with FANCM, another ATP-dependent translocase involved in DNA replication and genome stability. Their combined loss causes severe genome instability that we link to chromosome breakage at loci enriched in simple repeats, which are known to challenge replication fork progression. Our findings illuminate a critical genetic buffering mechanism that provides an essential function for maintaining genome integrity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


