感觉神经EP4通过调节血管生成耦合骨形成促进异位骨化
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
目的异位骨化(HO)是指骨骼在软组织内而非骨骼本身的异常发育。以往的研究表明,感觉神经前列腺素 E2 受体 4(EP4)信号不仅能控制痛觉,还能影响骨形成。然而,感觉神经EP4与跟腱HO发病机制之间的关系仍不清楚。本研究旨在探讨这种关系及其内在机制。方法我们繁殖了基因型为Avil-CreEP4fl/fl的感觉神经EP4特异性基因敲除小鼠。我们利用转录组测序和生物信息学分析技术确定了涉及感觉神经 EP4 的潜在分子通路。此外,还通过横断坐骨神经建立了神经切除小鼠模型,以研究体内外周神经支配对 HO 的影响和机制。研究采用显微 CT、免疫荧光 (IF)、苏木精和伊红 (H&E) 染色、沙弗宁 O-Fast Green 染色和 Western 印迹技术分析细胞和组织成分的变化。通过坐骨神经横断进行的近端神经切除术或靶向敲除感觉神经中的EP4阻碍了血管生成依赖性骨形成以及跟腱创伤部位HO的发展。此外,我们还发现Efnb2(Ephrin-B2)/Dll4(Delta-like ligand 4)轴是受感觉神经EP4影响的一个潜在下游元素,它在HO的调控过程中起着重要作用。值得注意的是,服用 EP4 抑制剂能够缓解 HO。结论我们的研究结果表明,感觉神经EP4在HO过程中通过调节血管生成相关的骨生成促进了异位骨的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
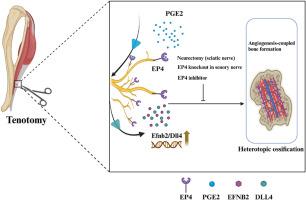
Sensory nerve EP4 facilitates heterotopic ossification by regulating angiogenesis-coupled bone formation
Objective
Heterotopic ossification (HO) refers to the abnormal development of bone in soft tissue rather than within bone itself. Previous research has shown that sensory nerve prostaglandin E2 receptor 4 (EP4) signaling not only governs pain perception but also influences bone formation. However, the relationship between sensory nerve EP4 and the pathogenesis of HO in the Achilles tendon remains unclear. This study aims to investigate this relationship and the underlying mechanisms.
Methods
We generated sensory nerve EP4-specific knockout mice, with the genotype of Avil-CreEP4fl/fl, was propagated. Transcriptome sequencing and bioinformatics analysis techniques were used to identify the potential molecular pathways involving with sensory nerve EP4. Additionally, a neurectomy mouse model was created by transecting the sciatic nerve transection, to examine the effects and mechanisms of peripheral innervation on HO in vivo. Micro-CT, immunofluorescence (IF), Hematoxylin and Eosin (H&E) Staining, Safranin O-Fast Green staining and western blotting were used to analyze changes in cellular and tissue components.
Results
We here observed an increase in sensory nerve EP4 and H-type vessels during the pathogenesis of HO in both human subjects and mice. Proximal neurectomy through sciatic nerve transection or the targeted knockout of EP4 in sensory nerves hindered angiogenesis-dependent bone formation and the development of HO at the traumatic site of the Achilles tendon. Furthermore, we identified the Efnb2 (Ephrin-B2)/Dll4 (Delta-like ligand 4) axis as a potential downstream element influenced by sensory nerve EP4 in the regulation of HO. Notably, administration of an EP4 inhibitor demonstrated the ability to alleviate HO. Based on these findings, sensory nerve EP4 emerges as an innovative and promising approach for managing HO.
Conclusion
Our findings demonstrate that the sensory nerve EP4 promotes ectopic bone formation by modulating angiogenesis-associated osteogenesis during HO.
The translational potential of this article
Our results provide a mechanistic rationale for targeting sensory nerve EP4 as a promising candidate for HO therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


