构建新型离子型 Co(II) 配位聚合物及其与 CNT 的复合材料,显示出对 AA 和 Fe3+ 的双重电化学传感能力
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用简便的缓慢蒸发法制备了一种新型离子钴(II)链配位聚合物{[Co(bbbm)(H2O)4]-ATBIP-2DMA-2H2O}n(Co-CP),该聚合物含有多功能 5-氨基-2,4,6-三溴间苯二甲酸(H2ATBIP)和柔性连接剂 1,4-双(苯并咪唑-1-甲基)苯(bbbm)。此外,还通过 "一锅 "合成法构建了其与羟基碳纳米管(CNTs)的复合材料(Co-CP@CNTs)。借助氢键带,Co-CP 进一步组装成具有一维通道的三维超分子框架。安培响应表明,Co-CP/GCE 和 Co-CP@CNTs/GCE 在 0.1 M H2SO4 中分别对抗坏血酸(AA)和 Fe3+ 具有灵敏和选择性的电化学氧化传感和还原传感。此外,与 Co-CP 相比,Co-CP@CNTs 对 AA 的氧化传感和 Fe3+ 的还原传感具有更好的电催化能力。值得注意的是,该策略已成功应用于便携式丝网印刷电极(SPE)。在三种电极中,Co-CP@CNTs/SPE 的传感速度最快,灵敏度最高,对 AA 和 Fe3+ 的指数范围分别为 0.01 - 26 mM 和 0.01 - 21 mM。Co-CP@CNTs/GCE 的检测限(LOD)分别达到 0.74 μM(AA)和 0.96 μM(Fe3+),Co-CP@CNTs/SPE 的检测限(LOD)分别达到 0.32 μM(AA)和 0.91 μM(Fe3+)。此外,便携式 Co-CP@CNTs/SPE 已成功用作实际检测真实世界样品中 AA 和 Fe3+ 的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
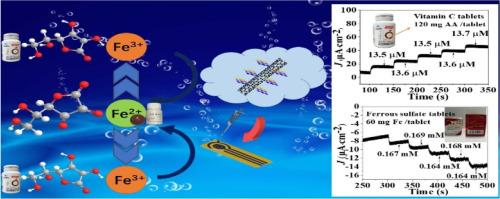
Construction of a new ionic Co(II) coordination polymer and its composite with CNTs showing dual electrochemical sensing to AA and Fe3+
A new ionic cobalt(II) chain coordination polymer, {[Co(bbbm)(H2O)4]·ATBIP·2DMA·2H2O}n (Co-CP) with the multi-functional 5-amino-2,4,6-tribromoisophthalic acid (H2ATBIP) and flexible linker 1, 4-bis(benzimidazole-1-methyl)benzene (bbbm) was prepared with convenient slow evaporation method. Moreover, its composite with hydroxyl carbon nanotubes (CNTs) (Co-CP@CNTs) was constructed by “one pot” synthesis. Co-CP further assembles into a 3D supramolecular framework with 1D channels with the aid of the hydrogen-bonded tape. The amperometric response demonstrates that Co-CP/GCE and Co-CP@CNTs/GCE show sensitive and selective electrochemical oxidation sensing to ascorbic acid (AA) and reduction sensing to Fe3+ in 0.1 M H2SO4, respectively. Moreover, Co-CP@CNTs exhibits better electrocatalytic capability for the oxidation sensing of AA and reduction sensing of Fe3+ than that of Co-CP. Remarkably, the strategy was successfully applied in the portable screen-printed electrode (SPE). Among three electrodes, the Co-CP@CNTs/SPE shows the most rapid and sensitive sensing with the largest exponential range of 0.01 - 26 mM for AA and 0.01 - 21 mM for Fe3+, respectively. The limits of detection (LOD) reach to 0.74 μM (AA) and 0.96 μM (Fe3+) for Co-CP@CNTs/GCE, 0.32 μM (AA) and 0.91 μM (Fe3+) for Co-CP@CNTs/SPE, respectively. Furthermore, the portable Co-CP@CNTs/SPE has been successfully used as a platform for the practical detection of AA and Fe3+ in the real-world samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


