锌(II)盐与 4-芳基-2-(吡啶-2-基)喹啉的络合研究:阴离子对配合物结构及其发光特性的影响
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
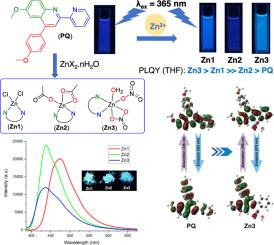
合成了一种新型配体 6-甲氧基-4-(4-甲氧基苯基)-2-(吡啶-2-基)喹啉 (PQ),并研究了它与 ZnX2.nH2O (X:Cl-、OAc-、NO3-)的相互作用,从而形成了三种类型的[Zn(X2)(PQ)(H2O)n](Zn1-Zn3)配合物,产率高达 75% 至 94%。利用 ESI 质谱、红外和 1H NMR 光谱以及单晶 X 射线衍射(Zn1 和 Zn3)对 Zn1-Zn3 的结构进行了全面鉴定。光谱分析显示,Zn1、Zn2 和 Zn3 中的中心原子 Zn(II)的配位数分别为 4、5 和 6。在这些配合物中,Zn(II)通过其两个氮原子与 PQ 配体配位,而其余的配位位点则被 Cl-、OAc、NO3- 等阴离子或 H₂O 所占据。研究结果还表明,Zn(II) 配合物的光学性质不仅取决于主有机配体 QP,还取决于次无机配体。在 THF 中,含有氯配体和硝基配体的配合物 Zn1 和 Zn3 在 443 和 462 纳米波长处发出强烈的蓝色荧光,量子产率分别为 0.66 和 0.70。虽然复合物 Zn2 在四氢呋喃中的发射强度最弱,但在固态下却表现出最强的蓝色发射强度。此外,计算研究深入揭示了其结构和电子特性,并与实验数据显示出很强的相关性。计算得出的吸收光谱和发射光谱主要基于高振荡强度的 HOMO 和 LUMO(π-π*)之间的转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A study on complexation of Zn(II) salts with 4-aryl-2-(pyridin-2-yl)quinolines: The influence of anions on the complex structures and their luminescent properties
A novel ligand, 6‑methoxy-4-(4-methoxyphenyl)-2-(pyridin-2-yl)quinoline (PQ) was synthesized and studied for its interaction with ZnX2.nH2O (X: Cl-, OAc-,) to form three complexes of the type [Zn(X2)(PQ)(H2O)n] (Zn1-Zn3) with high yields, ranging from 75 to 94 %. The structures of Zn1–Zn3 were fully characterized using ESI mass spectrometry, IR and 1H NMR spectroscopy, and single-crystal X-ray diffraction (for Zn1 and Zn3). Spectroscopic analyses revealed that Spectroscopic analyses revealed that the central atom Zn(II) in Zn1, Zn2, and Zn3 has the coordination numbers of 4, 5, and 6, respectively. In these complexes, Zn(II) coordinates with the PQ ligand through its two nitrogen atoms, while the remaining coordination sites are occupied by anions such as Cl-, OAc, , or by H₂O. The results also indicate that the optical properties of the Zn(II) complexes depend not only on the primary organic ligand QP but also on the secondary inorganic ligands. In THF, the complexes Zn1 and Zn3, which contain chlorido and nitrato ligands exhibit intense fluorescence blue emissions at 443 and 462 nm with quantum yields of 0.66 and 0.70, respectively. Although the complex Zn2 has the weakest emission in THF, it exhibited the strongest blue emission intensity in solid state. In addition, computational studies provided insights into the structural and electronic properties, showing a strong correlation with experimental data. The calculated absorption and emission spectra were promarily based on the transition between HOMO and LUMO (π-π*) with high oscillator strength.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


