变价 Co 基催化剂活化甲烷的机理研究
IF 3.1
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
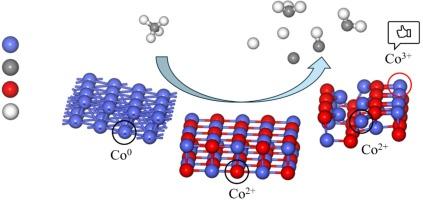
甲烷是一种重要的碳氢化合物气体,在能源生产和利用中发挥着关键作用。本研究利用密度泛函理论深入分析了甲烷在 Co (1 0 0) 、CoO (1 0 0) 和 Co3O4 (1 1 0) 表面的活化机理,揭示了钴价态不同导致的甲烷活化性能差异。在 Co(1 0 0)表面,甲烷脱氢主要通过直接脱氢和 O 辅助脱氢两种方式进行,其中 O 辅助脱氢的能障较高。CoO (1 0 0) 表面的能障明显高于 Co (1 0 0) 表面,因此不利于甲烷活化。相比之下,Co3O4 (1 1 0) 上甲烷解离和脱氢的能垒最低,Co3+ 的催化性能最好。此外,Co2+位点对甲烷的活化效应与CoO(1 0 0)表面上的类似,不如Co0和Co3+有效,这表明Co3O4(1 1 0)表面上的Co2+和Co3+在催化反应中并没有表现出明显的协同效应。这些研究成果有助于从原子水平揭示不同钴价态在甲烷活化中的机理作用,为设计高效的甲烷催化转化催化剂提供了重要指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study on the mechanism of methane activation on Co-based catalysts with variable valence
Methane is an important hydrocarbon gas that plays a key role in energy production and utilization. In this study, the mechanism of methane activation on Co (1 0 0), CoO (1 0 0), and Co3O4 (1 1 0) surfaces is thoroughly analyzed using density functional theory, revealing the performance differences in methane activation due to different valence states of cobalt. On the Co (1 0 0) surface, methane dehydrogenation mainly proceeds through direct dehydrogenation and O-assisted dehydrogenation, with the O-assisted dehydrogenation having a higher energy barrier. The energy barrier on the CoO (1 0 0) surface is significantly higher than that on the Co (1 0 0) surface, thus it is not favorable for methane activation. In contrast, the energy barrier for methane dissociation and dehydrogenation on Co3O4 (1 1 0) is the lowest, with Co3+ exhibiting the best catalytic performance. Additionally, the activation effect of Co2+ sites on methane is similar to that on the CoO (1 0 0) surface, and is less effective than Co0 and Co3+, indicating that the Co2+ and Co3+ on the Co3O4 (1 1 0) surface do not show a significant synergistic effect in catalytic reactions. These research findings help to reveal the mechanistic role of different cobalt valence states in methane activation at the atomic level, providing important guidance for the design of efficient methane catalytic conversion catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


