用于捕获重金属离子的聚(丙烯酸-2-羟乙基甲基丙烯酸酯)接枝树胶水凝胶
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
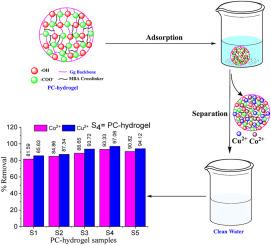
本研究探索了一种新型聚合物复合水凝胶(PC-hydrogel)的简便合成路线。首先根据傅立叶变换红外光谱、溶胀率(SR%)和表面负电荷(PZC)优化了甲基丙烯酸羟乙酯(HEMA)和丙烯酸(AA)的比例。结果表明,在自由基接枝共聚过程中,由 1:4 比例的 HEMA 和 AA 共聚物组成的 PC-水凝胶最适合接枝到 Gum ghatti(Gg)上。在所有其他可能的 HEMA: AA 组合中,1:4 比例接枝的 Gg 被称为 PC-hydrogel [聚(AA-co-HEMA)-g-Gg]。由于 AA 的增加,PC-水凝胶在很宽的 pH 值范围内都呈现出负表面电荷。制备的水凝胶在灰水、自来水和蒸馏水中的溶胀度(克/克)和保水率(%)分别为 342.6、385 和 412.6 克/克以及 74.83、65.30 和 57.86%。此外,PC-水凝胶还可用于捕捉水相中的 Cu2+ 和 Co2+ 离子。实验结果表明,吸附过程与 pH 值有关,在中性 pH 值为 7 时,Cu2+ 和 Co2+ 的吸附量最大。在 Langmuir、Freundlich 和 Temkin 等不同的吸附等温线模型中,实验数据与 Langmuir 吸附模型非常吻合,Cu2+ 和 Co2+ 的最大吸附容量分别为 381.67 和 328.94 mg/g。金属离子的捕获遵循伪二阶速率模型[Cu2+ 的速率常数 k = 1.7 x 10-4 和 Co2+ 的速率常数 k = 1.5 x 10-4 g/(mg.min)]。PC 水凝胶在连续三次吸附-解吸循环中都保持了对金属离子的吸附能力,并对 Cu2+、Co2+ 和其他(NaCl、MgCl2、CaCl2)共存离子表现出较高的选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Poly (acrylic acid-co-2-hydroxyethyl methacrylate)-grafted gum ghatti hydrogel for capturing heavy metal ions
In this work, a facile route is explored for the synthesis of a novel polymer composite-based hydrogel (PC-hydrogel). The ratio of 2-(Hydroxyethyl) methacrylate (HEMA) and acrylic acid (AA) is optimized first based on Fourier transform infra-red spectroscopy, swelling ratio (SR%) and surface negative charge (PZC). Results indicate that PC-hydrogel composed of copolymer of HEMA: AA in 1:4 ratio is optimized, for grafting on Gum ghatti (Gg) during free-radical graft copolymerization process. Among all other possible combination of HEMA: AA, 1:4 ratio grafted Gg is termed as PC-hydrogel [Poly (AA-co-HEMA)-g-Gg]. PC-hydrogel exhibited negative surface charge over a wide range of pH owing to increase in AA. The swelling (g/g) and water retention ratio (%) of the prepared hydrogel have been found to be 342.6, 385 & 412.6 g/g and 74.83, 65.30 & 57.86 % in grey, tap and distilled water respectively. Furthermore, PC-hydrogel is applied for capturing Cu2+ and Co2+ ions in aqueous phases. Experimental results showed that adsorption process was pH-dependent, and the maximum capturing of Cu2+ and Co2+ was observed at neutral pH 7. Among different adsorption isotherms models like Langmuir, Freundlich, and Temkin models, experimental data fitted closely with the Langmuir adsorption model showing a maximum adsorption capacity of 381.67 and 328.94 mg/g for Cu2+ and Co2+ respectively. The capturing of metal ion followed pseudo-second-order rate model [rate constant k = 1.7 x 10−4 for Cu2+ and 1.5 x 10−4 for Co2+ g/(mg.min)]. The PC-hydrogel property retained its uptake capacity of metal ions up to the three successive adsorption−desorption cycles, and exhibited higher selectivity towards Cu2+ and Co2+ and other (NaCl, MgCl2, CaCl2) coexisting ions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


