三钯络合物催化的炔烃半还原反应
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在氨硼烷的活化作用下,发现一种三钯复合物[(bpnp)2Pd3Cl2]Cl2(Pd3)对炔烃的氢化具有催化活性,可生成相应的顺式烯烃。通常情况下,将二苯基乙炔(0.80 mmol)、Pd3(0.2 mol %)和 NH3.BH3 (10 mol %)在 THF/H2O (10:1,1 mL)中的混合物在 30 °C、H2(1 atm)条件下搅拌 2 小时,得到 (Z) - 二苯乙烯,收率为 95%,翻转频率 (TOF) ∼ 240 h-1。这种催化剂适用于带有芳基或烷基取代基的内部和末端炔烃。本文章由计算机程序翻译,如有差异,请以英文原文为准。
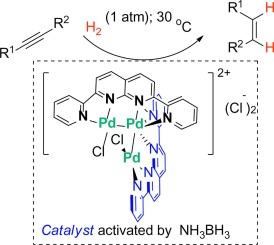
Semi-reduction of alkynes catalyzed by a tri-palladium complex
Upon activation by ammonia-borane, a tri-palladium complex [(bpnp)2Pd3Cl2]Cl2 (Pd3) was found to be catalytically active for hydrogenation of alkynes to render the corresponding cis-olefins. Typically, a mixture of diphenylacetylene (0.80 mmol), Pd3 (0.2 mol %), and NH3.BH3 (10 mol%) in THF/H2O (10:1, 1 mL) was stirred at 30 °C under H2 (1 atm) for 2 h, giving (Z)-stilbene in 95 % yield with a turnover frequency (TOF) ∼240 h−1. This catalyst is applicable to both internal and terminal alkynes with aryl or alkyl substituents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


