利用有机叠氮化物作为氨基源合成伯胺的区域和对映体选择性镍催化异位和远程氢化反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of the American Chemical Society
Pub Date : 2024-10-23
DOI:10.1021/jacs.4c1232410.1021/jacs.4c12324
引用次数: 0
摘要
伯胺是大多数其他含 N 化合物的关键合成前体,在有机化学和药物化学中非常重要。在此,我们介绍了一种 NiH 催化的温和同位和远位氢化技术,该技术利用有机叠氮化物作为可脱保护的伯胺来源。这一策略为高效构建 α-手性支链伯胺和线性伯胺提供了一个高度灵活的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
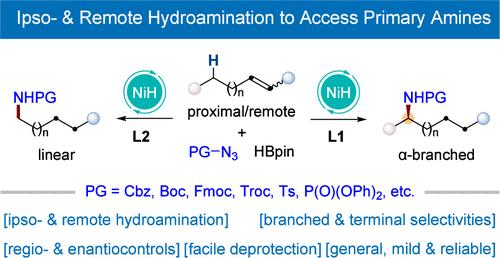
Regio- and Enantioselective Nickel-Catalyzed Ipso- and Remote Hydroamination Utilizing Organic Azides as Amino Sources for the Synthesis of Primary Amines
Primary amines serve as key synthetic precursors to most other N-containing compounds, which are important in organic and medicinal chemistry. Herein, we present a NiH-catalyzed mild ipso- and remote hydroamination technique that utilizes organic azides as deprotectable primary amine sources. This strategy offers a highly flexible platform for the efficient construction of α-chiral branched primary amines, as well as linear primary amines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


