芳基硼酸酯中的杂质会引发持久余辉
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
最近的几份报告表明,芳基硼酸酯可以表现出室温磷光(RTP),这种光学特性在防伪印刷、氧传感和生物成像等应用中非常理想。这些发现对有机化合物中发生系统间交叉需要重元素或轨道对称性变化这一基本概念提出了挑战。由于我们多年来合成的许多芳基硼酸酯中都没有观察到长余辉,因此我们怀疑在这些体系中观察到的 RTP 有更简单的解释:报告的材料不纯。在此,我们合成了 12 种以前报道过的显示 RTP 的芳基硼酸酯,并通过柱层析、重结晶和升华等方法对它们进行了仔细的纯化。我们结合单晶 X 射线衍射分析和详细的理论研究,重新研究了它们的光物理特性。虽然这 12 种化合物中有 4 种在粗产物中显示出较长的余辉,但经过仔细纯化后,它们都没有显示出持久的 RTP。我们还成功地分离出了杂质 4-氨基-3,5-双(频哪酮酰基)苯甲腈 (2),并确定它就是导致 3,5-双(频哪酮酰基)苯甲腈 (1) 产生延迟荧光的杂质。在 1 中掺入 1.0 摩尔%的 2 会导致持续的余辉,其寿命为 67 毫秒,这是由二聚体电荷转移态介导的。我们的发现要求人们重新审视以前报告芳基硼酸酯产生 RTP 的研究,强调了光物理研究中仔细纯化的重要性,并为设计具有长余辉的有机材料提供了实用策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
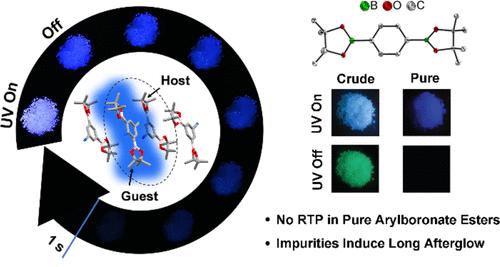
Impurities in Arylboronic Esters Induce Persistent Afterglow
Several recent reports suggest that arylboronic esters can exhibit room temperature phosphorescence (RTP), an optical property that is desirable for applications in security printing, oxygen sensing, and bioimaging. These findings challenged the fundamental notion that heavy elements or changes in orbital symmetry were required for intersystem crossing to occur in organic compounds. As we had not observed long afterglow in the many arylboronic esters we had synthesized over many years, we suspected that the RTP observed in these systems had a simpler explanation: the materials reported were impure. Herein, we synthesized 12 arylboronic esters that were previously reported to show RTP, and carefully purified them by column chromatography, recrystallization, and sublimation. We re-examined their photophysical properties alongside single-crystal X-ray diffraction analysis and detailed theoretical studies. While 4 of the 12 compounds showed long afterglows as crude products, none of them showed persistent RTP after careful purification. We also successfully isolated the impurity 4-amino-3,5-bis(pinacolatoboryl)benzonitrile (2), identifying it as the impurity responsible for inducing delayed fluorescence in 3,5-bis(pinacolatoboryl)benzonitrile (1). Doping 1 with 1.0 mol % 2 led to a persistent afterglow with a lifetime of 67 ms, which is mediated by a dimer charge transfer state. Our findings call for a re-examination of previous studies reporting RTP from arylboronic esters, highlight the importance of careful purification in photophysical research, and provide a practical strategy for designing organic materials with a long afterglow.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


