含有乳清酸和异乳清酸的铀络合物的合成与表征
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
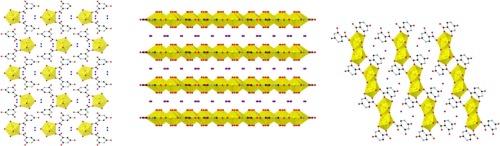
三种新的铀有机框架(UOFs)--([UO2(C5H3N2O4)2(H2O)3]-2H2O (1)、(H3O)3[(UO2)4(C5HN2O4)(O)4](2)、和 H3O[UO2(C5H2N2O4)(OH)](3),其中铀酰(UO22+)以不同的配位模式和齿度配位到乳清酸盐(1 和 2)或异乳清酸盐(3)上。络合物 1 是通过从混合溶剂(乙醇-水)中缓慢蒸发制备的,而络合物 2 和 3 则是通过水热法制备的。单晶 X 射线衍射分析表明,络合物 1 为 0D 结构,络合物 2 为 2D 结构,络合物 3 为 1D 结构。在每个复合物中,铀都呈现出近似五边形的双锥几何结构,这是线性二氧铀酰单元在赤道区域与水、乳清酸盐/异乳清酸盐和/或其他铀酰单元配位的结果。观察到的乳清酸盐(在 1 中)和异乳清酸盐(在 3 中)的配位模式与之前的报道一致。然而,在复合物 2 中,乳清酸配体通过所有可能的供体原子(两个羧基氧原子、两个羰基氧原子和两个氮原子)结合在一起。这是首个以这种方式配位的乳清酸配体实例。本文介绍了这些新配合物的合成、表征和结构描述。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and characterization of uranyl complexes containing orotate and isoorotate
The synthesis and structural characterization of three new Uranyl Organic Frameworks (UOFs) – ([UO2(C5H3N2O4)2(H2O)3]·2H2O (1), (H3O)3[(UO2)4(C5HN2O4)(O)4] (2), and H3O[UO2(C5H2N2O4)(OH)] (3) – in which uranyl ( is coordinated to either orotate (1 and 2) or isoorotate (3) with varying coordination modes and denticities, is presented. Complex 1 was prepared via slow evaporation from a mixed solvent (ethanol–water), while complexes 2 and 3 were prepared via a hydrothermal methodology. Single crystal X-ray diffraction analysis reveals that 1 is a 0D structure, 2 is a 2D structure, and 3 is a 1D structure. In each complex, uranium exhibits an approximately pentagonal bipyramidal geometry, a result of the linear dioxo uranyl unit being coordinated by water, orotate/isoorotate, and/or other uranyl units in the equatorial region. The observed coordination mode(s) of orotate (in 1) and isoorotate (in 3) are consistent with what has previously been reported. However, in complex 2, the orotate ligand is bound through every possible donor atom (two carboxylate oxygens, two carbonyl oxygens, and two nitrogen atoms). This is the first example of orotate coordinated in this manner. Herein, the synthesis, characterization, and structural descriptions of these new complexes is presented.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


