利用落叶合成多功能磁性活性炭,以活化过硫酸盐降解酸性红 18
IF 2.218
Q2 Chemistry
引用次数: 0
摘要
在这里,磁性活性炭(MAC)是通过一锅热解含FeCl3和ZnCl2的落叶松(FSLs)制备的。结果发现了不同的金属基纳米颗粒(Fe(0)、FeO、Fe3O4 和 ZnO),并均匀地分布在活性炭(AC)框架中。MAC 的 SBET 高达 479 m2/g,Vtotal 大至 0.30 cm3/g,磁分离 MS 为 3.71 emu/g。在应用中,0.100 g/L MAC 可激活初始 pH 值为 3.0 的 2.00 mM 过硫酸盐(PS),在 60 分钟内快速消除 96.5 ± 0.4 % 的 50 ppm 酸性红 18(AR18)。此外,淬灭实验表明,在处理 AR18 的过程中,MAC+PS 系统可能会产生活性硫酸根 (SO4--) 和羟基 (-OH) 自由基。总之,这些研究结果凸显了多功能 FSL 衍生 MAC 的潜力,因为它具有磁性分离性和有效的 PS 激活能力,可用于降解 AR18。本文章由计算机程序翻译,如有差异,请以英文原文为准。
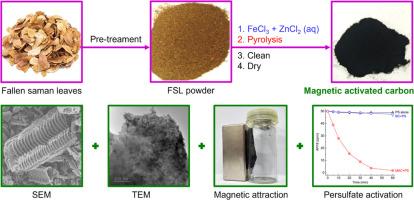
One-pot synthesis of multifunctional magnetic activated carbon from fallen saman leaves to activate persulfate for acid red 18 degradation
Herein, magnetic activated carbon (MAC) was prepared via one-pot pyrolysis of FeCl3- and ZnCl2-loaded fallen saman leaves (FSLs). As a result, different metal-based nanoparticles (Fe(0), FeO, Fe3O4, and ZnO) were identified and evenly distributed within the activated carbon (AC) framework. MAC had a high SBET of 479 m2/g, a large Vtotal of 0.30 cm3/g, and a MS of 3.71 emu/g for magnetic separation. For application, 0.100 g/L MAC activated 2.00 mM persulfate (PS) at initial pH 3.0 to rapidly eliminate 96.5 ± 0.4 % of 50 ppm acid red 18 (AR18) within 60 min. Furthermore, quenching experiments indicated that the MAC+PS system might produce both reactive sulfate (SO4•−) and hydroxyl (•OH) radicals during AR18 treatment. Summarily, the findings highlighted the potential of multifunctional FSL-derived MAC due to its magnetic separability and effective PS activation ability towards AR18 degradation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Data Collections
Chemistry-Chemistry (all)
CiteScore
6.10
自引率
0.00%
发文量
169
审稿时长
24 days
期刊介绍:
Chemical Data Collections (CDC) provides a publication outlet for the increasing need to make research material and data easy to share and re-use. Publication of research data with CDC will allow scientists to: -Make their data easy to find and access -Benefit from the fast publication process -Contribute to proper data citation and attribution -Publish their intermediate and null/negative results -Receive recognition for the work that does not fit traditional article format. The research data will be published as ''data articles'' that support fast and easy submission and quick peer-review processes. Data articles introduced by CDC are short self-contained publications about research materials and data. They must provide the scientific context of the described work and contain the following elements: a title, list of authors (plus affiliations), abstract, keywords, graphical abstract, metadata table, main text and at least three references. The journal welcomes submissions focusing on (but not limited to) the following categories of research output: spectral data, syntheses, crystallographic data, computational simulations, molecular dynamics and models, physicochemical data, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


