掺碳和非贵金属装饰的 p-BN2 在吸附 H2S 和 SO2 气体中的作用
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们使用五边形氮化硼 penta-BN2(p-BN2)表面作为气体传感器应用的挑战性材料。我们还研究了在表面添加掺杂剂的效果,以及用非贵金属装饰的效果。密度泛函理论(DFT)计算用于研究原始(p-BN2)和掺杂碳上吸附的 H2S 和 SO2 气体的几何和电子结构。结果表明,原始表面的解吸过程非常容易,而且检测率很高。这表明这些表面适合用作 H2S 和 SO2 气体的传感器。此外,原始表面还装饰了镍(Ni)、铁(Fe)和钴(Co)。此外,还研究了气体在经过装饰的金属原始表面上的吸附能及其电子特性。结果表明,吸附作用(化学吸附)更强;也就是说,气体和表面之间的电荷转移大大增加。因此,这些表面更适合捕获气体和通过分解去除气体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
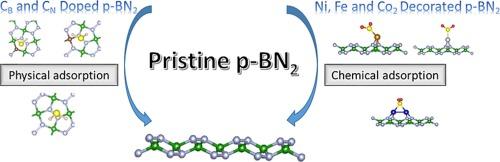
The role of carbon doped and non-noble metal decorated p-BN2 for the adsorption of H2S and SO2 gases
We used pentagonal boron nitride penta-BN2 (p-BN2) surface as a challenging material for the application as a gas sensor. The effect of applying a dopant on the surface was also studied, alongside the effect of decoration with a non-noble metal. Density Functional Theory (DFT) calculations were used to investigate the geometrical and electronic structures of the adsorbed H2S and SO2 gases on pristine (p-BN2) and doped carbon. They showed easy desorption processes from pristine surface and lead to high detection rates. This indicates that these surfaces are suitable for being used as sensors for H2S and SO2 gases. Additionally, pristine was decorated with nickel (Ni), iron (Fe), and cobalt (Co). The adsorption energies of the gases on the decorated metal pristine and their electronic properties were also performed. The results showed stronger adsorptions (chemisorption); that is, the charge transfers between the gas and the surface was considerably increased. These surfaces are, hence, more suitable for gases capture and removal by decomposition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


