Nampt/SIRT2/LDHA通路介导的乳酸生成调节多囊卵巢综合征的卵泡发育不良。
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
烟酰胺腺嘌呤二核苷酸(NAD+)含量的降低已被证明会导致衰老过程中的代谢功能障碍,包括多囊卵巢综合症(PCOS)。然而,NAD+通过调节糖酵解对多囊卵巢综合征排卵功能障碍的影响尚未见报道。根据基因表达总库(GEO)对颗粒细胞(GCs)转录组数据的观察,包括糖酵解和烟酸-烟酰胺代谢在内的信号通路在多囊卵巢综合征患者中明显富集,且上述通路的大多数基因如LDHA和SIRT2在多囊卵巢综合征患者中下调。因此,我们通过来曲唑与高脂饮食(HFD)结合建立了多囊卵巢综合征大鼠模型,证明体内补充烟酰胺单核苷酸(NMN)可通过促进卵泡发育、促进黄体形成以及提高多囊卵巢综合征大鼠的生育能力来明显改善排卵功能障碍。此外,目标能量代谢组学和转录组结果显示,补充 NMN 可通过激活卵巢中的糖酵解过程来改善乳酸的产生。在体外,当 NAD+ 合成和 SIRT2 表达受到抑制时,KGN 细胞中的乳酸含量降低,LDHA 表达受到显著抑制。我们通过共免疫沉淀(Co-IP)试验证实,FK866 能增强 293T 细胞上 LDHA 的乙酰化。我们还观察到,抑制 NAD+ 合成可降低 KGN 细胞的活性并增加其凋亡。总之,我们阐明了 NMN 的这些益处,Nampt/SIRT2/LDHA 途径介导的颗粒细胞乳酸生成在改善多囊卵巢综合征的卵泡发育障碍中发挥了重要作用。这项研究将为未来 NMN 在治疗多囊卵巢综合征方面的临床应用提供实验证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
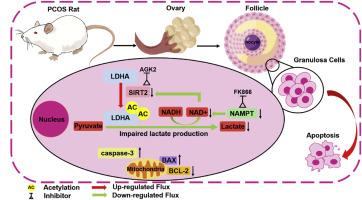
Nampt/SIRT2/LDHA pathway-mediated lactate production regulates follicular dysplasia in polycystic ovary syndrome
Decreased nicotinamide adenine dinucleotide (NAD+) content has been shown to contribute to metabolic dysfunction during aging, including polycystic ovary syndrome (PCOS). However, the effect of NAD+ on ovulatory dysfunction in PCOS by regulating glycolysis has not been reported. Based on the observations of granulosa cells (GCs) transcriptome data from the Gene Expression Omnibus (GEO) database, the signal pathways including glycolysis and nicotinate-nicotinamide metabolism were significantly enriched, and most genes of the above pathway like LDHA and SIRT2 were down-regulated in PCOS patients. Therefore, the PCOS rat model was established by combining letrozole with a high-fat diet (HFD), we demonstrate that in vivo supplementation of nicotinamide mononucleotide (NMN) significantly improves the ovulatory dysfunction by facilitating the follicular development, promoting luteal formation, as well the fertility in PCOS rats. Furthermore, target energy metabolomics and transcriptome results showed that NMN supplementation ameliorates the lactate production by activating glycolytic process in the ovary. In vitro, when NAD+ synthesis and SIRT2 expression were inhibited, lactate content in KGN cells was decreased and LDHA expression was significantly inhibited. We confirmed that FK866 can enhance the acetylation of LDHA on 293T cells by Co-immunoprecipitation (Co-IP) assay. We also observed that inhibition of NAD+ synthesis can reduce the activity and increase the apoptosis of KGN cells. Overall, these benefits of NMN were elucidated and the Nampt/SIRT2/LDHA pathway mediated lactate production in granulosa cells played an important role in the improvement of follicular development disorders in PCOS. This study will provide experimental evidence for the clinical application of NMN in the treatment of PCOS in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


