环氧替格连内酯通过经典的蛋白激酶 C 激活诱导角质形成细胞的伤口愈合反应,从而促进皮肤再上皮化。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
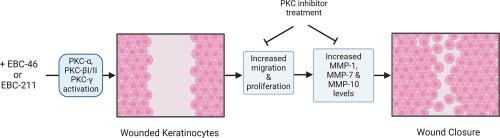
环氧替格连酯是一类新型的二萜酯。环氧替格连酯的原型 EBC-46(替吉烷醇替吉烷酸酯)是一种强效抗癌剂,目前正处于临床开发阶段,可用于一系列人类和动物肿瘤的局部治疗。EBC-46 还通过激活经典的蛋白激酶 C(PKC)同工酶,持续促进治疗部位的伤口再上皮化。我们以前的研究表明,环氧替格连内酯可刺激体外永生化人类皮肤角质细胞(HaCaTs)的增殖和伤口再填充反应,而泛 PKC 抑制剂双吲哚马来酰亚胺-1 则可抑制这种反应。在本研究中,我们进一步研究了用 1.51 nM-15.1 µM EBC-46 或类似物 EBC-211 处理 HaCaT 后,负责诱导这种伤口愈合反应的特定 PKC 同工酶。用 GӦ6976 (1 μM)对 PKC 进行经典抑制,可显著减少所有环氧替吉利安浓度下环氧替吉利安诱导的 HaCaT 增殖和伤口再填充。用恩杂陶灵(1 μM)抑制 PKC-βI/-βII 同工酶,可明显抑制两种环氧替格连内酯诱导的 HaCaT 增殖和伤口再增殖反应,尤其是在 1.51-151 nM 的浓度下。PKC-α 抑制剂甲磺酸 Ro 31-8220(10 nM)对 HaCaT 反应的抑制作用较小。关键角蛋白(KRT17)和细胞周期(细胞周期蛋白 B1、CDKN1A)蛋白水平的环氧三烯酸变化在一定程度上受到 GӦ6976 和 enzastaurin 的抑制。GӦ6976还抑制了基质金属蛋白酶(MMP-1、MMP-7、MMP-10)活性的增加。磷酸化 PKC(p-PKC)研究证实,环氧替格连内酯能以剂量和时间依赖性的方式瞬时激活经典的 PKC 异构体(p-PKCα、p-PKC-βI/-βII、p-PKCγ)。通过确定环氧替格连内酯如何刺激经典 PKC 促进角朊细胞愈合反应和再上皮化,这些研究结果支持进一步将环氧替格连内酯开发成局部治疗药物,用于治疗皮肤伤口不愈合等再上皮化受损的临床情况。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Epoxytiglianes induce keratinocyte wound healing responses via classical protein kinase C activation to promote skin re-epithelialization
Epoxytiglianes are a novel class of diterpene esters. The prototype epoxytigliane, EBC-46 (tigilanol tiglate), is a potent anti-cancer agent in clinical development for local treatment of a range of human and animal tumors. EBC-46 also consistently promotes wound re-epithelialization at the treatment sites, mediated via activation of classical protein kinase C (PKC) isoforms. We have previously shown that epoxytiglianes stimulate proliferative and wound repopulation responses in immortalized human skin keratinocytes (HaCaTs) in vitro, abrogated by pan-PKC inhibitor, bisindolylmaleimide-1. In this study, we further investigate the specific PKC isoforms responsible for inducing such wound healing responses, following HaCaT treatment with 1.51 nM-15.1 µM EBC-46 or analogue, EBC-211. Classical PKC inhibition by GӦ6976 (1 μM), significantly attenuated epoxytigliane induced, HaCaT proliferation and wound repopulation at all epoxytigliane concentrations. PKC-βI/-βII isoform inhibition by enzastaurin (1 μM), significantly inhibited HaCaT proliferation and wound repopulation responses induced by both epoxytiglianes, especially at 1.51–151 nM. PKC-α inhibitor, Ro 31–8220 mesylate (10 nM), exerted lesser inhibitory effects on HaCaT responses. Epoxytigliane changes in key keratin (KRT17) and cell cycle (cyclin B1, CDKN1A) protein levels were partly attenuated by GӦ6976 and enzastaurin. GӦ6976 also inhibited increases in matrix metalloproteinase (MMP-1, MMP-7, MMP-10) activities. Phospho-PKC (p-PKC) studies confirmed that epoxytiglianes transiently activated classical PKC isoforms (p-PKCα, p-PKC-βI/-βII, p-PKCγ) in a dose- and time-dependent manner. By identifying how epoxytiglianes stimulate classical PKCs to facilitate keratinocyte healing responses and re-epithelialization, these findings support further epoxytigliane development as topical therapeutics for clinical situations involving impaired re-epithelialization, such as non-healing wounds in skin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


