柠檬酸乙酰三丁酯可减轻 5-氟尿嘧啶诱导的人类角朊细胞炎症、氧化应激和细胞凋亡。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
5-氟尿嘧啶(5-FU)是一种常用的化疗药物,能有效摧毁癌细胞。尽管这种药物被广泛使用且疗效显著,但它也带来了相当大的挑战,尤其是对快速分裂的正常细胞(如角质形成细胞)的不利影响。这些不利影响可归因于炎症、氧化和凋亡潜能,从而导致严重的皮肤疾病。由于缺乏针对 5-FU 引起的皮肤病副作用的特效疗法,人们只能采用传统疗法,但这些疗法的缓解效果有限,而且存在缺陷。本研究调查了柠檬酸乙酰三丁酯(ATBC)对 5-FU 处理的人类角质细胞的影响。研究结果表明,ATBC 大大减少了 5-FU 引起的炎症,核因子卡巴 B 的核转位及其下游基因(包括肿瘤坏死因子、白细胞介素 1 beta(IL1B)和 IL6)的表达均证明了这一点。ATBC 还能显著降低氧化应激,这体现在 5-FU 处理细胞中的活性氧水平和超氧化物歧化酶 1 (SOD1)、SOD2 和血红素加氧酶 1 等抗氧化基因的表达上。此外,通过乳酸脱氢酶释放和附件素 V/碘化丙啶流式细胞术测定,ATBC 可减轻 5-FU 诱导的细胞凋亡,这可能与干扰素相关基因的参与有关。随后,蛋白激酶 C delta 被预测为 ATBC 可能的分子靶点。这些发现建议将 ATBC 作为一种治疗药物,用于控制与 5-FU 治疗相关的皮肤副作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
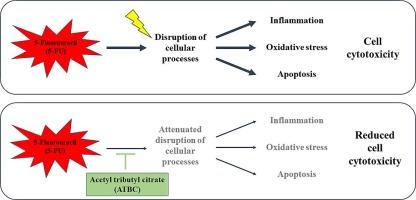
Acetyl tributyl citrate attenuates 5-fluorouracil-induced inflammation, oxidative stress, and apoptosis in human keratinocytes
5-Fluorouracil (5-FU) is a commonly used chemotherapy drug that effectively destroys cancer cells. Despite its widespread use and efficacy, it also presents considerable challenges, particularly with adverse effects on rapidly dividing normal cells, such as keratinocytes. These detrimental effects are attributed to inflammatory, oxidative, and apoptotic potentials, leading to severe skin disorders. Due to the lack of specific remedies for 5-FU-induced dermatological side effects, conventional treatments are applied instead, which provide limited relief and have drawbacks. This study investigated the impact of acetyl tributyl citrate (ATBC) in 5-FU-treated human keratinocytes. The findings indicated that ATBC substantially reduced inflammation caused by 5-FU, as demonstrated by nuclear translocation of nuclear factor kappa B and expression of its downstream genes, including tumor necrosis factor, interleukin 1 beta (IL1B), and IL6. ATBC also markedly decreased oxidative stress, indicated by reactive oxygen species levels and the antioxidant gene expression such as superoxide dismutase 1 (SOD1), SOD2, and heme oxygenase 1 in 5-FU-treated cells. Furthermore, ATBC attenuated 5-FU-induced apoptosis, as determined by lactate dehydrogenase release and Annexin V/propidium iodide flow cytometry, with the potential involvement of interferon-related genes. Following this, protein kinase C delta was predicted as a possible molecular target of ATBC. These findings propose ATBC as a therapeutic agent for managing the cutaneous side effects associated with 5-FU treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


