调节小鼠体内牛磺酸平衡的独特机制:营养供应会影响肝脏中的牛磺酸水平,而能量限制则会影响肠道中的牛磺酸水平。
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
目的:我们之前的研究结果表明,热量限制(CR)会刺激小鼠产生和分泌牛磺酸结合胆汁酸。随后,肠道微生物群的处理会导致肠道中脱结合胆汁酸、牛磺酸和各种牛磺酸共轭物水平的增加。此外,我们还证明,碳水化合物限制和蛋白质限制在较小程度上反映了 CR 对肝脏产生胆汁酸而非分泌胆汁酸的影响。我们假设,调节膳食中的宏量营养素水平将影响自由采食动物和CR动物肝脏和肠道中的牛磺酸平衡:将自由采食的雄性小鼠分配为对照组、低蛋白(LP)组、低脂肪(LF)组或低碳水化合物(LC)组。同时,给CR组提供80%的常规自愿食物摄入量,作为对照、高蛋白(HP)、高脂肪(HF)或高碳水化合物(HC)饮食:主要研究结果:虽然 CR 不会影响肝脏中的牛磺酸水平及其共轭物,但碳水化合物和蛋白质摄入量的改变会对其产生影响。相反,在肠道中,CR 增加了游离牛磺酸和共轭牛磺酸的含量,而各种饮食并不影响游离牛磺酸和共轭牛磺酸的含量,也不会破坏 CR 特异性表型。值得注意的是,日粮组成的变化影响了肠道中牛磺酸转运体(Slc6a6)和谷胱甘肽-S 转移酶(GST)以及肝脏中半胱氨酸二氧酶(Cdo)的表达:肝脏和肠道对饮食干预表现出不同的反应,肝脏牛磺酸受饮食成分的影响,而肠道牛磺酸则受能量供应的制约。本文章由计算机程序翻译,如有差异,请以英文原文为准。
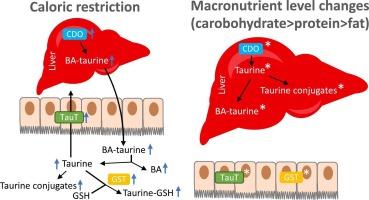
The distinct mechanism regulating taurine homeostasis in mice: Nutrient availability affects taurine levels in the liver and energy restriction influences it in the intestine
Aims
Our previous findings indicate that caloric restriction (CR) stimulates the production and secretion of taurine-conjugated bile acids in mice. Subsequent processing by gut microbiota leads to increased levels of deconjugated bile acids, taurine, and various taurine conjugates in the intestine. Furthermore, we demonstrated that carbohydrate restriction and protein restriction, to a smaller extent, mirror the impact of CR in terms of hepatic production of bile acids but not their secretion. We hypothesized that modulating dietary macronutrient levels would influence taurine homeostasis in the liver and intestine of ad libitum-fed and CR animals.
Materials and methods
Ad libitum-fed male mice were allocated to receive either a control, low-protein (LP), low-fat (LF), or low-carbohydrate (LC) diet. Meanwhile, CR groups were given 80 % of their regular voluntary food intake as a control, high-protein (HP), high-fat (HF), or high-carbohydrate (HC) diet.
Key findings
While CR did not affect the taurine levels and its conjugates in the liver, alteration in carbohydrates and protein intake impacted it. Conversely, in the intestine, CR increased the amount of free and conjugated taurine, whereas the various diets did not affect it or disrupt the CR-specific phenotype. Notably, variations in diet composition impacted the expression of the taurine transporter (Slc6a6) and glutathione-S transferases (GST) in the intestine as well as cysteine dioxygenase (Cdo) in the liver.
Significance
The liver and the intestine show distinct responses to dietary interventions, with hepatic taurine being affected by the diet composition, while intestinal taurine is governed by energy availability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


