脂肪量和肥胖相关蛋白通过 miR-320-3p/SLC7A11 轴抑制铁蛋白沉积减轻脑缺血再灌注损伤
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
脂肪量与肥胖相关蛋白(FTO)是一种去甲基化酶,最近发现它对急性缺血性中风(AIS)有保护作用,但其潜在机制在很大程度上还不清楚。新的研究发现,某些 miRNA 的表达可能受 N6-甲基腺苷(m6A)水平的影响。在此,我们利用高通量测序和定量聚合酶链反应发现,miR-320-3p 在 AIS 患者中显著上调;miR-320-3p 会加重大脑中动脉闭塞/再灌注模型小鼠的神经行为表现、梗死体积和组织病理学。在机制上,miR-320-3p 与溶质运载家族 7 成员 11(SLC7A11)mRNA 的 3' 非翻译区结合,促进氧化应激和缺氧-葡萄糖/复氧诱导的神经元铁蛋白沉积。FTO 可抑制主转录本 pri-miR-320 的 m6A 甲基化和 miR-320-3p 的成熟,从而对 AIS 后的脑缺血再灌注损伤具有保护作用。在临床上,我们也证实了 AIS 患者外周血中 FTO 和 SLC7A11 mRNA 的下调及其与 miR-320-3p 表达的相关性。我们的研究发现,FTO通过miR-320-3p/SLC7A11轴以m6A依赖的方式抑制铁氧化,从而对脑缺血再灌注损伤具有保护作用。我们的研究结果为 AIS 后脑缺血再灌注损伤提供了一个很有前景的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
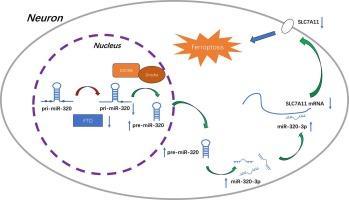
Fat mass and obesity-associated protein alleviates cerebral ischemia/reperfusion injury by inhibiting ferroptosis via miR-320-3p/SLC7A11 axis
Fat mass and obesity-associated protein (FTO) is a demethylase and has recently been found to have a protective effect in acute ischemic stroke (AIS), but the underlying mechanism is unclear to a large extent. New studies have found that the expression of certain miRNAs may be affected by N6-methyladenosine (m6A) levels. Here, using high-throughput sequencing and quantitative polymerase chain reaction, we found miR-320-3p was significantly up-regulated in AIS patients. miR-320-3p aggravated the neurobehavioral manifestation, infarct volume and histopathology of middle cerebral artery occlusion/reperfusion model mice. Mechanically, miR-320-3p binds to the 3′ untranslated region of solute carrier family 7 member 11 (SLC7A11) mRNA, promoting oxidative stress and ferroptosis induced by oxygen-glucose deprivation/reoxygenation in neurons. FTO inhibited the m6A methylation of the primary transcript pri-miR-320 and the maturation of miR-320-3p, thus having a protective effect on cerebral ischemia/reperfusion injury after AIS. Clinically, we also confirmed the down-regulation of FTO and SLC7A11 mRNA in the peripheral blood of AIS patients and their correlation with the expression of miR-320-3p. Our study found that FTO inhibits ferroptosis through miR-320-3p/SLC7A11 axis in an m6A-dependent manner, and thus has a protective effect on cerebral ischemic reperfusion injury. Our results provided a promising therapeutic target of cerebral ischemia/reperfusion injury after AIS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


