基于肿瘤微环境响应型 PtAu/MnO2 级联纳米反应器的多功能集成纳米平台,具有多种酶活性,可用于多模式肿瘤协同治疗
IF 9.4
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用或改善肿瘤微环境(TME)已成为新兴肿瘤疗法的一个突破口。为了解决单一疗法疗效有限的问题,我们开发了一种多功能集成纳米平台,该平台基于具有多酶活性的TME响应型PtAu/MnO2级联纳米反应器,可用于多模式协同肿瘤治疗。PtAu/MnO2级联纳米反应器利用TME中的微酸性环境和高水平谷胱甘肽(GSH),消耗GSH,然后还原生成锰离子(Mn2+)并释放PtAu纳米颗粒(NPs)。随后,多模式肿瘤协同疗法被激活。首先,GSH 的耗竭抑制了谷胱甘肽过氧化物酶 4 的活性,导致脂质过氧化物的积累,从而诱导肿瘤细胞铁中毒。其次,PtAu NPs 具有过氧化氢酶样、葡萄糖氧化酶样和烟酰胺腺嘌呤二核苷酸(NADH)氧化酶样活性,为级联反应生成氧气,缓解缺氧,并进一步耗竭葡萄糖、NADH 和三磷酸腺苷,通过饥饿疗法抑制肿瘤细胞增殖。第三,PtAu NPs 的氧化酶和过氧化物酶样活性以及 Mn2+ 的芬顿样反应产生的活性氧同时通过化学动力学疗法诱导肿瘤细胞凋亡。简而言之,体外和体内研究结果证实,基于具有五种纳米酶活性的 PtAu/MnO2 级联纳米反应器的多功能集成纳米平台具有出色的生物相容性,并能通过协同铁氧化/饥饿疗法/细胞凋亡抑制肿瘤生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
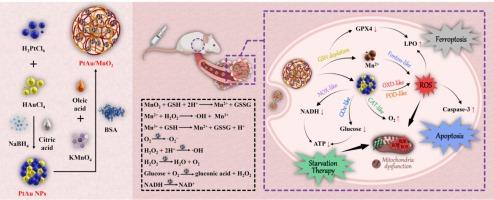
A multi-functional integrated nanoplatform based on a tumor microenvironment-responsive PtAu/MnO2 cascade nanoreactor with multi-enzymatic activities for multimodal synergistic tumor therapy
The utilization or improvement of tumor microenvironment (TME) has become a breakthrough in emerging oncology therapies. To address the limited therapeutic efficacy of single modality, a multi-functional integrated nanoplatform based on a TME-responsive PtAu/MnO2 cascade nanoreactor with multi-enzymatic activities was developed for multimodal synergistic tumor therapy. Benefiting from the slightly acidic environment and high-level glutathione (GSH) in TME, PtAu/MnO2 cascade nanoreactor consumed GSH, followed by the reductive generation of manganese ion (Mn2+) and the release of PtAu nanoparticles (NPs). Then, the multimodal synergistic tumor therapy was activated as follows. First, GSH depletion inhibited the activity of glutathione peroxidase 4 and led to the accumulation of lipid peroxidation, thereby inducing tumor cell ferroptosis. Second, PtAu NPs exhibited catalase-like, glucose oxidase-like and nicotinamide adenine dinucleotide (NADH) oxidase-like activities, which generated oxygen for the cascade reaction to alleviate hypoxia and further depleted glucose, NADH and adenosine triphosphate, leading to the inhibition of tumor cell proliferation via starvation therapy. Third, the production of reactive oxygen species by the oxidase- and peroxidase-like activities of PtAu NPs and the Fenton-like reaction of Mn2+ simultaneously induced tumor cell apoptosis via chemodynamic therapy. Briefly, the in vitro and in vivo results confirmed that the multi-functional integrated nanoplatform based on a PtAu/MnO2 cascade nanoreactor with five nanozyme activities demonstrated outstanding biocompatibility and greater inhibition of tumor growth via synergistic ferroptosis/starvation therapy/apoptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


