Ni-N4、相邻单金属原子和 Fe6 纳米粒子提供的多金属位点对促进二氧化碳活化和还原的协同效应
IF 9.4
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
嵌入具有 M-Nx-C 构型的掺氮碳材料中的单过渡金属 (TM) 原子已成为一类很有前途的电化学二氧化碳还原 (CO2RR) 电催化剂。然而,在 TM 原子密度较高的情况下,全面了解活性位点结构和反应机理仍是一项重大挑战,但这对提高 CO2RR 性能至关重要。在这项工作中,我们利用第一性原理计算研究了 Ni-N4 位点在邻近 TM 原子和 Fe6 纳米粒子的共同协助下将 CO2 还原成 CO 的电催化性能。与之前研究的许多保持线性 CO2 结构的 Ni-N4 催化剂不同,相邻 TM 原子和 Fe6 的结合可诱导 Ni 位点上的 CO2 发生弯曲和活化,增强其质子化以形成关键的 *COOH 中间体,同时保持高效的 *CO 解吸。新设计的混合电催化剂展示了多金属位点在促进 CO2 还原成 CO 方面的协同效应。具体来说,TM 原子促进了 Ni 位点与 *CO2/*COOH 物种之间 C-Ni 键的形成,而 Fe6 则形成了 Fe...O 配位键。对反应机理和能量学的详细分析表明,Ni-N4 在单个 TM 原子和 Fe6(尤其是 TM = Ni、Cu 或 Ag)的共同辅助下,在 -0.5 V 的低极限电位下,对 CO 的生成表现出更强的催化活性。这项研究提出了一种在高金属含量条件下提高单原子催化剂催化活性的有效策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
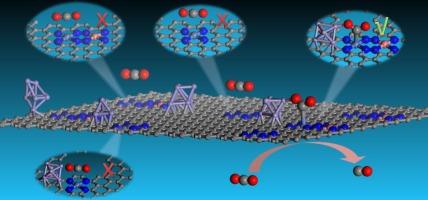
Synergistic effect of multi-metal site provided by Ni-N4, adjacent single metal atom, and Fe6 nanoparticle to boost CO2 activation and reduction
Single transition metal (TM) atom embedded in nitrogen-doped carbon materials with M−Nx−C configuration have emerged as a promising class of electrocatalysts for electrochemical CO2 reduction (CO2RR). However, at high TM atom densities, a comprehensive understanding of the active site structure and reaction mechanisms remains a significant challenge, yet it is crucial for enhancing CO2RR performance. In this work, we use first-principles calculations to investigate the electrocatalytic performance of Ni-N4 sites for CO2 reduction to CO, co-assisted by neighboring TM atoms and a Fe6 nanoparticle. Unlike many previously studied Ni-N4 catalysts that maintain a linear CO2 structure, the combination of adjacent TM atoms and Fe6 induces bending and activation of CO2 at the Ni site, enhancing its protonation to form key *COOH intermediate while maintaining efficient *CO desorption. The newly designed hybrid electrocatalyst demonstrates a synergistic effect of multi-metal sites in boosting CO2 reduction to CO. Specifically, the TM atom facilitates C–Ni bond formation between the Ni site and *CO2/*COOH species, while Fe6 forms an Fe…O coordination bond. Detailed analysis of reaction mechanisms and energetics show that Ni-N4, co-assisted by a single TM atom and Fe6 (especially TM = Ni, Cu, or Ag), exhibits enhanced catalytic activity for CO production with a low limiting potential of −0.5 V. This work presents an effective strategy for improving the catalytic activity of single-atom catalysts (SACs) at high metal content.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


