多细胞肿瘤球:癌症转化研究的便捷体外模型
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
为了减少单层培养结果对癌症治疗靶点和化合物鉴定的不确定性,人们对使用三维肿瘤球体模型的兴趣与日俱增,这些模型包括肿瘤球体(TS)、组织衍生肿瘤球体(TDTS)、器官型多细胞肿瘤球体(OMS)和多细胞肿瘤球体(MCTS)。与其他球状模型相比,多细胞肿瘤球(MCTSs),无论是单细胞肿瘤球(Mono-MCTSs)还是异细胞肿瘤球(Hetero-MCTSs),无论是有支架还是无支架,都具有许多优点,包括易于培养、可重复性高、可获得性强、通量高、大小可控、形状圆润、遗传操作简单、经济实惠以及可利用各种生物学方法进行开发。在这篇综述中,我们试图讨论 MCTS 在癌症转化研究的各个方面所发挥的作用,如药物反应和渗透、细胞-细胞相互作用以及侵袭和转移。然而,无论是不含支架还是基于支架的单一 MCTS,都可能无法充分代表临床肿瘤的细胞异质性和复杂性,从而限制了它们在癌症转化研究中的应用。相反,无支架和基于支架的异种 MCTS 模型由于存在类似的体内肿瘤微环境,因此显示出更好的适用性。不过,基于支架的异种-MCTS 模型显示出批次变异性,并且由于难以从支架中提取球体和细胞,在进行定量测定时面临挑战。此外,将基质细胞与癌细胞以更精确的比例结合在一起开发异种-MCTS,可以增强模型的相关性,为候选药物带来更可靠的临床结果,并提高对肿瘤生物学的洞察力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
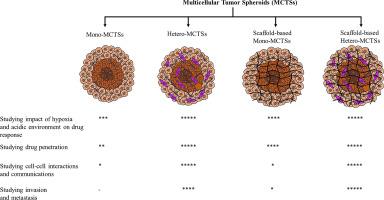
Multicellular tumor spheroids: A convenient in vitro model for translational cancer research
In the attempts to mitigate uncertainties in the results of monolayer culture for the identification of cancer therapeutic targets and compounds, there has been a growing interest in using 3D cancer spheroid models, which include tumorospheres (TSs), tissue-derived tumor spheres (TDTSs), organotypic multicellular tumor spheroids (OMSs), and multicellular tumor spheroids (MCTSs). The MCTSs, either Mono-MCTSs or Hetero-MCTSs, with or without scaffold, in particular, offer numerous advantages over other spheroid models, including easy cultivation, high reproducibility, accessibility, high throughput, controllable size, well-rounded shape, simplicity of genetic manipulation, economical and availability of various biological methods for their development. In this review, we have attempted to discuss the role of MCTSs concerning various aspects of translational cancer research, such as drug response and penetration, cell-cell interaction, and invasion and metastasis. However, the Mono-MCTSs, either scaffold-free or scaffold-based, may not adequately represent the cellular heterogeneity and complexity of clinical tumors, limiting their utility in translational cancer research. Conversely, Hetero-MCTS models, both scaffold-free and scaffold-based, show better suitability due to the presence of a similar in vivo type tumor microenvironment. Nonetheless, scaffold-based Hetero-MCTS models show batch variability and challenges in performing quantitative assays due to difficulties extracting spheroids and cells from scaffolds. Further, incorporating stromal cells with cancer cells in a more precise ratio to develop Hetero-MCTSs can enhance the model's relevance, yielding more clinically reliable outcomes for drug candidates and improving insights into tumor biology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


