具有聚集诱导发射的环金属化铱(III)-三丁基锡(IV)羧酸席夫碱配合物的抗癌行为
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
环金属化的铱(III)和有机锡(IV)羧酸盐配合物已在抗癌领域显示出潜在的应用价值。然而,这些配合物普遍存在的聚集诱导淬灭(ACQ)效应不利于对其靶向性和抗癌机理的探索,而聚集诱导发射(AIE)效应的观点可以有效解决这一问题。因此,本研究设计并制备了AIE激活的环金属化铱(III)-三丁基锡(IV)羧酸席夫碱配合物。配合物在高浓度溶液或聚集状态下表现出 AIE 效应,这为研究亚细胞组织靶向(线粒体)和细胞形态提供了便利。与环金属化铱(III)配合物和三丁基锡(IV)羧酸单体相比,这些配合物对 A549 细胞显示出更好的体外抗增殖活性,证实了它们具有良好的协同抗癌活性。即使是对 A549/DDP(顺铂抗性)细胞,这些复合物也表现出更好的活性。此外,这些复合物还显示出线粒体凋亡途径。因此,环金属化铱(III)-三丁基锡(IV)羧酸席夫碱配合物可作为铂类药物的潜在替代品,并获得进一步的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
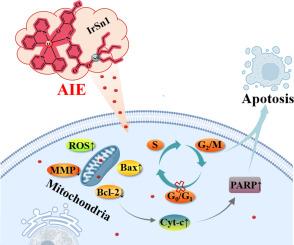
Anticancer behavior of cyclometallated iridium(III)-tributyltin(IV) carboxylate schiff base complexes with aggregation-induced emission
Cyclometallated iridium(III) and organotin(IV) carboxylate complexes have shown potential application value in the field of anticancer. However, the widespread aggregation-caused quenching (ACQ) effect of these complexes is not conducive to the exploration of their targeting and anticancer mechanism, and the idea of aggregation-induced emission (AIE) effect can effectively solve this problem. Then, AIE-activated cyclometallated iridium(III)-tributyltin(IV) carboxylate Schiff base complexes were designed and prepared in this study. Complexes exhibited AIE effect in highly concentrated solution or aggregative state, which facilitated the investigation of subcellular tissue targeting (mitochondria) and cell morphology. Compared with cyclometallated iridium(III) complex and tributyltin(IV) carboxylate monomers, these complexes showed the better in-vitro anti-proliferative activity toward A549 cells, confirming the favorable synergistic anticancer activity. Even for A549/DDP (cisplatin-resistance) cells, these complexes also exhibited the better activity. In addition, complexes showed a mitochondrial apoptotic pathway. Therefore, cyclometallated iridium(III)-tributyltin(IV) carboxylate Schiff base complexes can be used as the potential substitutes for platinum-based drugs and gain further application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


