甲状腺激素受体 beta(THRβ1)是人类 iPSC 衍生肝细胞中 T3 作用的主要调节因子。
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
目的:甲状腺激素(TH)的作用是由甲状腺激素受体(THR)同工酶介导的。虽然 THRβ1 可能是肝脏中表达的主要同工酶,但其在人类肝细胞中的作用尚未完全明了:为了阐明THRβ1在人类肝细胞中的作用,我们使用CRISPR/Cas9编辑技术敲除诱导多能干细胞(iPSC)中的THRβ1。在定向分化为肝系后,我们对 iPSC 衍生的肝细胞进行了检测,以确定 THRβ1 在配体依赖性和配体依赖性功能中的作用:结果:我们发现,THRβ1的缺失促进了增殖率和受T3调控的代谢途径的改变,包括葡萄糖生成、脂质氧化、脂肪酸合成和脂肪酸摄取。我们观察到,参与肝脏代谢的关键基因通过依赖于 T3 配体和不依赖于 THRβ1 的信号机制进行调控。最后,我们证明了在THRβ1基因敲除后,几个关键的代谢基因仍然对T3有反应,这表明它们是THRα的靶标:这些结果突出表明,iPSC 衍生的肝细胞是研究人类肝细胞中 TH 信号调节机制的有效平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
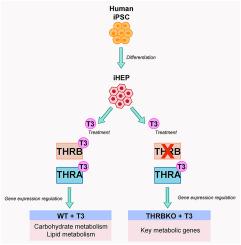
Thyroid hormone receptor beta (THRβ1) is the major regulator of T3 action in human iPSC-derived hepatocytes
Objective
Thyroid hormone (TH) action is mediated by thyroid hormone receptor (THR) isoforms. While THRβ1 is likely the main isoform expressed in liver, its role in human hepatocytes is not fully understood.
Methods
To elucidate the role of THRβ1 action in human hepatocytes we used CRISPR/Cas9 editing to knock out THRβ1 in induced pluripotent stem cells (iPSC). Following directed differentiation to the hepatic lineage, iPSC-derived hepatocytes were then interrogated to determine the role of THRβ1 in ligand-independent and -dependent functions.
Results
We found that the loss of THRβ1 promoted alterations in proliferation rate and metabolic pathways regulated by T3, including gluconeogenesis, lipid oxidation, fatty acid synthesis, and fatty acid uptake. We observed that key genes involved in liver metabolism are regulated through both T3 ligand-dependent and -independent THRβ1 signaling mechanisms. Finally, we demonstrate that following THRβ1 knockout, several key metabolic genes remain T3 responsive suggesting they are THRα targets.
Conclusions
These results highlight that iPSC-derived hepatocytes are an effective platform to study mechanisms regulating TH signaling in human hepatocytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


