吲哚-3-乙酸通过调节肺部微生物群、抑制成纤维细胞活化和缓解肺泡上皮细胞衰老来减轻肺纤维化。
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
目的:肺纤维化(PF)是一种无情的渐进性疾病,其特点是死亡率高且有效的治疗方案有限。吲哚-3-乙酸(IAA)最初被认为是一种植物激素,但在哺乳动物体内也被确认为一种从微生物群分解而来的色氨酸衍生代谢物。IAA 在多种疾病中表现出抗氧化、抗炎和抗肿瘤作用,但其在 PF 中的作用仍然难以捉摸:主要方法:使用博莱霉素(BLM)在小鼠模型中诱导 PF。主要方法:利用博莱霉素(BLM)在小鼠模型中诱导 PF,利用 TGF-β1 在原代小鼠肺成纤维细胞(pMLFs)中建立促纤维化体外细胞模型,并在 A549 细胞中建立体外细胞衰老模型。通过苏木精-伊红染色、免疫荧光染色、Western 印迹、SA-β-gal 检测和网络药理学分析,评估了 IAA 对肺纤维化的治疗效果。此外,我们还利用 16S rRNA 基因测序分析研究了 IAA 对 PF 肺部微生物群的影响。此外,我们还证明了 IAA 在 BLM 诱导的小鼠模型中缓解 PF 的治疗潜力,并显示出剂量依赖性反应。从机理上讲,我们探讨了三个方面。首先,IAA通过抑制PI3K/AKT/mTOR通路促进自噬通量,从而抑制肺成纤维细胞分化和细胞外基质(ECM)沉积。其次,IAA 通过调节 PI3K/AKT 和 HIF-1 通路,减轻肺泡上皮细胞的衰老。最后,IAA通过调节肺部微生物群的结构和组成,显示出缓解肺泡上皮细胞衰老的能力:我们的研究表明,IAA能通过多种途径缓解肺水肿,这凸显了它作为一种治疗药物的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
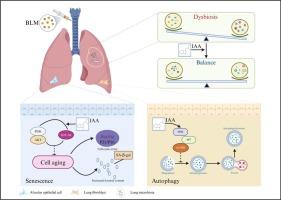
Indole-3-acetic acid attenuates pulmonary fibrosis by modulating lung microbiota, inhibiting fibroblast activation, and alleviating alveolar epithelial cell senescence
Aim
Pulmonary fibrosis (PF) is a relentlessly progressive disorder characterized by high mortality and limited effective therapeutic options. Indole-3-acetic acid (IAA), originally recognized as a plant hormone, is also identified as a tryptophan-derived metabolite catabolized from microbiota in mammals. IAA has exhibited antioxidative, anti-inflammatory, and anti-tumor effects in various disorders, yet its role in PF remains elusive.
Main methods
Bleomycin (BLM) was employed to induce PF in a mouse model. TGF-β1 was utilized in primary mouse lung fibroblasts (pMLFs) to establish a pro-fibrotic in vitro cellular model, and in A549 cells to create an in vitro cellular senescence model. The therapeutic effects of IAA on PF were evaluated using hematoxylin-eosin staining, immunofluorescence staining, western blotting, SA-β-gal assay, and network pharmacology analysis. Additionally, the effect of IAA on lung microbiota of PF was investigated using 16S rRNA gene sequencing analysis.
Key findings
we observed a significant reduction in IAA levels in both PF patients and mouse models. Moreover, we demonstrated the therapeutic potential of IAA in alleviating PF in BLM-induced mouse models, showing a dose-dependent response. Mechanistically, we delineated three perspectives. Firstly, IAA promoted autophagic flux by inhibiting the PI3K/AKT/mTOR pathway, thereby suppressing lung fibroblast differentiation and extracellular matrix (ECM) deposition. Secondly, IAA attenuated alveolar epithelial cell senescence by modulating the PI3K/AKT and HIF-1 pathways. Lastly, IAA displayed the ability to mitigate PF by modulating the structure and composition of lung microbiota.
Significance
Our study demonstrates that IAA alleviates PF through multiple pathways, highlighting its potential as a therapeutic agent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


