电压和 Ca2+ 诱导的 PLC 活性,用于分析爪蟾卵母细胞中离子通道对 PI(4,5)P2 的敏感性。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
磷脂酰肌醇 4,5-二磷酸(PIP2)是调节各种离子通道活动的关键膜脂。目前,有多种分子工具可用于调节 PIP2 的水平,但每种工具都有各自的优缺点。在本研究中,我们提出了一种利用异源爪蟾卵母细胞的新方法,即利用能水解 PIP2 的磷脂酶 C(PLC)-ζ 来精确操纵 PIP2 水平。注射了 PLCζ 的爪蟾卵母细胞在 Ca2+ 驱动力增加的驱动下表现出明显的超极化诱导 Ca2+ 流入。PLCζ 对 Ca2+ 的高敏感性促进了超极化诱导的 PLC 在章鱼卵母细胞中的活性,这种活性是电压和 Ca2+ 依赖性的。这项研究证明了 PLCζ 以电压和 Ca2+ 依赖性方式调节 PIP2 敏感离子通道(如 KCNQ2/3 和 GIRK 通道)的调节能力。此外,PLCζ的激活途径只需要一个双电极电压钳装置,这使其成为一种方便的分子工具,可与电压感应磷酸酶(VSP)相结合来操纵 PIP2 水平。与 VSP 相比,PLCζ 具有明显的特点和优势:(1) 超极化(而非去极化)会降低 PIP2 水平;(2) PIP2 水平降低的同时,磷脂酰肌醇 4-单磷酸(PIP)水平不会增加;(3) 施加 Ca2+ 会降低 PIP2 水平。因此,PLCζ 有效地支持了对 PIP2 如何与 VSP 一起调节离子通道的理解。总之,本研究强调了 PLCζ 的独特性及其在分析异种卵母细胞中 PIP2 和 PLC 通路对离子通道调控的独特优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
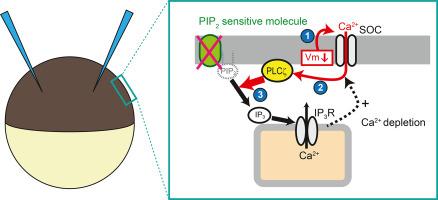
Voltage- and Ca2+-inducible PLC activity for analyzing PI(4,5)P2 sensitivity of ion channels in Xenopus oocytes
Phosphatidylinositol 4,5-bisphosphate (PIP2) is a key membrane lipid regulating various ion channel activities. Currently, several molecular tools are used to modulate PIP2 levels, each of which has distinct advantages and drawbacks. In this study, we proposed a novel methodology using heterologous Xenopus oocytes to precisely manipulate PIP2 levels using phospholipase C (PLC)-ζ, which hydrolyzes PIP2. Xenopus oocytes injected with PLCζ exhibited notable hyperpolarization-induced Ca2+ influx driven by the increased driving force of Ca2+. High Ca2+ sensitivity of PLCζ facilitated hyperpolarization-induced PLC activity in Xenopus oocytes that was voltage- and Ca2+-dependent. This study demonstrated the regulatory capacity of PLCζ in modulating PIP2-sensitive ion channels, such as the KCNQ2/3 and GIRK channels, in a voltage- and Ca2+-dependent manner. Moreover, activation pathway of PLCζ only requires a two-electrode voltage clamp setup, making it a convenient molecular tool to manipulate PIP2 levels in combination with a voltage-sensing phosphatase (VSP). PLCζ has distinct characteristics and advantages compared to VSP: (1) Hyperpolarization, but not depolarization, reduced the PIP2 levels, (2) PIP2 levels were decreased without any increase in phosphatidylinositol 4-monophosphate (PIP) levels, and (3) PIP2 levels were reduced by Ca2+ administration. Therefore, PLCζ effectively supports understanding how PIP2 regulates ion channels, alongside VSP. Overall, this study highlights the unique characteristics of PLCζ and its distinct advantages in analyzing ion channel regulation by PIP2 and the PLC pathway in Xenopus oocytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


