洞察宿主-肠道微生物黄嘌呤协同代谢对高脂饮食喂养小鼠的调节作用。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
肠道微生物群介导的内生物和外生物代谢在疾病进展和药物治疗/毒性方面发挥着至关重要的作用。我们最近的研究表明,肠道微生物群介导的黄嘌呤代谢与抵抗高脂饮食(HFD)诱导的肥胖相关。在此,我们通过口服长双歧杆菌、黄嘌呤和黄嘌呤氧化酶抑制剂(托吡罗司他),探讨了宿主-肠道微生物黄嘌呤协同代谢在预防和治疗高脂饮食诱发肥胖中的作用。研究结果表明,黄嘌呤对高氟酸诱导的肥胖具有明显的保护作用。长春花酵母菌、黄嘌呤和托吡咯司他并不能缓解高频分解诱导肥胖(DIO)和肥胖抵抗(DIR)小鼠体重和糖代谢紊乱。16S rRNA测序分析表明,使用长春花酵母菌治疗可显著改变HFD喂养小鼠和DIO小鼠的肠道微生物群组成。微生物相互作用网络分析揭示了几个类杆菌属物种,如Amulumruptor caecigallinarius和Muribaculum intestinale,它们是关键的类群,被龙胆球菌明显富集。非靶向代谢组学分析表明,黄嘌呤可能是调节体重的关键分子,对高氟酸诱导的肥胖具有预防作用。这项研究为研究宿主-肠道微生物黄嘌呤协同代谢对HFD喂养小鼠的影响提供了新的视角,并强调了黄嘌呤在促进体重减轻方面的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
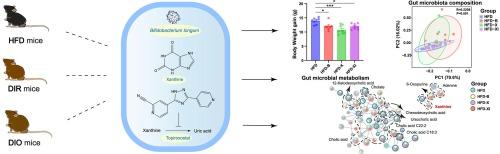
Insights into the modulatory effects of host-gut microbial xanthine co-metabolism on high-fat diet-fed mice
Gut microbiota-mediated endobiotic and xenobiotic metabolism play crucial roles in disease progression, and drug therapy/toxicity. Our recent study suggested that gut microbiota-mediated xanthine metabolism is correlated with resistance to high-fat diet (HFD)-induced obesity. Here, we explored the role of host-gut microbial xanthine co-metabolism in the prevention and treatment of HFD-induced obesity by orally administration of Bifidobacterium longum, xanthine, and a xanthine oxidase inhibitor (topiroxostat). The findings indicate that xanthine exhibits a significantly protective effect against HFD-induced obesity. While B. longum, xanthine, and topiroxostat did not alleviate the dysbiosis of the weight and glucose metabolism of HFD-induced obesity (DIO) and obesity resistance (DIR) mice. 16S rRNA sequencing analyses revealed that treatments with B. longum significantly altered gut microbiota composition in HFD-fed and DIO mice. Microbial interaction network analysis revealed several Bacteroidetes species, such as Amulumruptor caecigallinarius and Muribaculum intestinale, as keystone taxa that were notably enriched by B. longum. Untargeted metabolomics analysis implied that xanthine might serve as a crucial molecule in regulating body weight, exerting a preventive effect on HFD-induced obesity. This study offers new perspectives on the influence of host-gut microbial xanthine co-metabolism on HFD-fed mice and emphasizes the promising role of xanthine in promoting weight loss
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


