通过对映选择性钯催化烯丙基取代策略全合成(-)-天冬苷
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报告了通过对映选择性 Pd 催化烯丙基取代策略全合成 (-)-aspidospermidine 的过程。这是 Pd 催化的烯丙基取代 3-取代吲哚衍生物在合成 Aspidosperma 生物碱中的首次应用。在我们的合成路线中,烯丙基取代反应是立体决定性步骤。然后以完全非对映选择性的顺序构建五环框架。最终,我们用七个线性步骤完成了迄今为止所报道的最短的对映体选择性合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
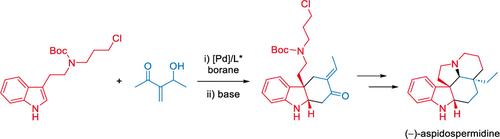
Total Synthesis of (−)-Aspidospermidine via an Enantioselective Palladium-Catalyzed Allylic Substitution Strategy
A total synthesis of (−)-aspidospermidine via an enantioselective Pd-catalyzed allylic substitution strategy is reported. This represents the first application of a Pd-catalyzed allylic substitution with a 3-substituted indole derivative in the synthesis of Aspidosperma alkaloids. In our synthetic route, the allylic substitution reaction was the stereo defining step. The pentacyclic framework was then constructed in a fully diastereoselective sequence. This culminated in the shortest enantioselective synthesis of aspidospermidine reported to date, in seven linear steps.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


