新辅助维杜莫德和 nivolumab 治疗高风险可切除黑色素瘤:前瞻性 II 期试验
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
瘤内TLR9激动剂和抗PD-1可产生临床反应和广泛的免疫激活。我们开展了一项新辅助TLR9激动剂维杜莫德联合抗PD-1 nivolumab治疗高风险可切除黑色素瘤的单臂研究。在31例可评估的患者中,观察到55%的主要病理反应(MPR),达到了主要终点。MPR与肿瘤坏死、噬黑素细胞增多、肿瘤微环境中CD8+肿瘤浸润淋巴细胞和浆细胞树突状细胞(pDCs)增多以及外周Ki67+CD8+ T细胞增多有关。MPRs在治疗前具有丰富的骨髓细胞基因特征,对治疗的反应与免疫细胞、pDCs、吞噬和巨噬细胞活化的基因特征有关。MPRs的肠道微生物群富含属于类杆菌科和肠杆菌科的革兰氏阴性菌以及革兰氏阴性固醇菌小亚群。我们的研究结果表明,维杜莫德和 nivolumab 能激发广泛的抗肿瘤免疫反应,并与不同的基线髓系基因特征和肠道微生物群相关。ClinicalTrials.gov identifier:NCT03618641。本文章由计算机程序翻译,如有差异,请以英文原文为准。
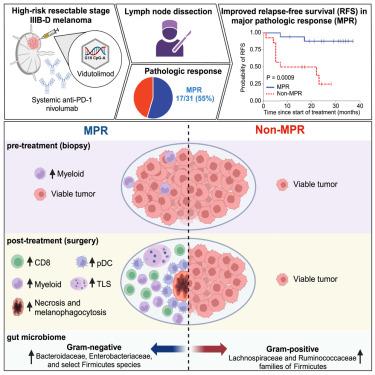
Neoadjuvant vidutolimod and nivolumab in high-risk resectable melanoma: A prospective phase II trial
Intratumoral TLR9 agonists and anti-PD-1 produce clinical responses and broad immune activation. We conducted a single-arm study of neoadjuvant TLR9 agonist vidutolimod combined with anti-PD-1 nivolumab in high-risk resectable melanoma. In 31 evaluable patients, 55% major pathologic response (MPR) was observed, meeting primary endpoint. MPR was associated with necrosis, and melanophagocytosis with increased CD8+ tumor-infiltrating lymphocytes and plasmacytoid dendritic cells (pDCs) in the tumor microenvironment, and increased frequencies of Ki67+CD8+ T cells peripherally. MPRs had an enriched pre-treatment gene signature of myeloid cells, and response to therapy was associated with gene signatures of immune cells, pDCs, phagocytosis, and macrophage activation. MPRs gut microbiota were enriched for Gram-negative bacteria belonging to the Bacteroidaceae and Enterobacteriaceae families and the small subgroup of Gram-negative Firmicutes. Our findings support that combined vidutolimod and nivolumab stimulates a broad anti-tumor immune response and is associated with distinct baseline myeloid gene signature and gut microbiota. ClinicalTrials.gov identifier: NCT03618641.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


