利用静态混合器作为气液接触器,建立臭氧辅助铈氧化还原过程的数学模型,用于不锈钢部件的表面去除
IF 3.3
3区 工程技术
Q1 NUCLEAR SCIENCE & TECHNOLOGY
引用次数: 0
摘要
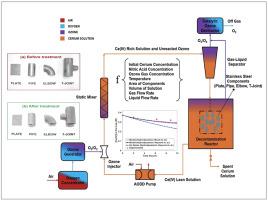
本文论述了一种基于铈的氧化还原化学工艺,该工艺用于去除和净化核设施运行和退役过程中产生的不锈钢部件的表面。该过程包括用高度氧化的 Ce(IV)离子腐蚀受污染的金属表面(10-50 μm),以清除夹带的放射性核素。金属表面氧化后产生的还原 Ce(III)离子会被臭氧持续氧化回 Ce(IV)。在这项工作中,建立了一个集合参数模型,该模型考虑了钢成分将 Ce(IV)还原为 Ce(III)以及臭氧在静态混合器中将 Ce(III)氧化为 Ce(IV)的情况。使用不同几何形状的非放射性 AISI SS304L 不锈钢部件,即板材、管材、弯头和 T 型接头(总表面积为 348 cm2),在室温 4 M HNO3 中 0.4 M 的 Ce(IV) 溶液中进行 5 L 规模的腐蚀实验,对模型进行了实验验证。利用经过验证的模型,对试验性金属去污设施进行了设计模拟,以说明工艺参数对环路中平衡 Ce(IV)浓度的影响,该浓度对部件腐蚀和去污至关重要。模拟结果表明,对于给定的初始铈盐浓度,增加气体/液体流速、臭氧浓度、初始硝酸浓度和溶液体积可降低平衡铈(IV)浓度随时间的下降速度。然而,温度和组分表面积的增加会提高 Ce(IV)平衡浓度随时间的下降速度。此外,还发现硝酸的酸度降低会限制组分的处理时间。此外,选择 Ce(III)或 Ce(IV)盐作为铈离子源,在使用 Ce(IV)的臭氧再生时,长期来看对金属部件的腐蚀没有影响。在各种几何形状的部件中,具有弯曲几何形状或焊接接头的部件的腐蚀率相对较高。焊接和非焊接部件的扫描电子显微镜图像显示,臭氧化 Ce(IV)溶液会导致晶间腐蚀,这可能是从金属表面去除放射性核素的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mathematical modeling of ozone assisted cerium redox process for the surface removal of stainless steel components using a static mixer as gas-liquid contactor
This paper deals with a cerium based redox chemical process which is used for the surface removal and decontamination of stainless steel components arising from the operation and decommissioning of nuclear facilities. The process involves the corrosion of top contaminated metal surface (∼10–50 μm) by highly oxidizing Ce(IV) ions, in order to remove the entrapped radionuclides. The reduced Ce(III) ions thus generated after the oxidation of metal surface are continuously oxidized back to Ce(IV) by ozone. In this work, a lumped parameter model, accounting for the reduction of Ce(IV) to Ce(III) by steel components and the simultaneous oxidation of Ce(III) to Ce(IV) by ozone in a static mixer, has been developed. The model is experimentally validated by carrying out a corrosion experiment at 5 L scale in ∼0.4 M solution of Ce(IV) in 4 M HNO3 at room temperature, by using non-radioactive AISI SS304L stainless steel components of different geometries viz. plate, pipe, elbow and T-Joint with a total surface area of 348 cm2. Using the validated model, design simulations of a pilot metal decontamination facility are carried out, to illustrate the effect of process parameters on the equilibrium Ce(IV) concentration in the loop, which is critical to component corrosion and decontamination. Simulation results show that, for a given initial cerium salt concentration, increasing the gas/liquid flow rate, ozone concentration, initial nitric acid concentration, and solution volume reduces the rate of fall of equilibrium Ce(IV) concentration with time. However, an increase in the temperature and surface area of the components enhances the rate of fall of equilibrium concentration of Ce(IV) with time. Additionally, reducing acidity of nitric acid has been found to limit the treatment time of components. Furthermore, the choice of a Ce(III) or Ce(IV) salt, as a source of cerium ions, has been shown to have no effect on the corrosion of metal components in a long run, when ozone regeneration of Ce(IV) is employed. Among the components of various geometries, relatively higher corrosion rates have been observed for the components with a curved geometry or a weld joint. SEM images of the welded and non-welded components show the occurrence of intergranular corrosion due to ozonated Ce(IV) solution, which is the likely mechanism for the removal of radionuclides from the metal surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Progress in Nuclear Energy
工程技术-核科学技术
CiteScore
5.30
自引率
14.80%
发文量
331
审稿时长
3.5 months
期刊介绍:
Progress in Nuclear Energy is an international review journal covering all aspects of nuclear science and engineering. In keeping with the maturity of nuclear power, articles on safety, siting and environmental problems are encouraged, as are those associated with economics and fuel management. However, basic physics and engineering will remain an important aspect of the editorial policy. Articles published are either of a review nature or present new material in more depth. They are aimed at researchers and technically-oriented managers working in the nuclear energy field.
Please note the following:
1) PNE seeks high quality research papers which are medium to long in length. Short research papers should be submitted to the journal Annals in Nuclear Energy.
2) PNE reserves the right to reject papers which are based solely on routine application of computer codes used to produce reactor designs or explain existing reactor phenomena. Such papers, although worthy, are best left as laboratory reports whereas Progress in Nuclear Energy seeks papers of originality, which are archival in nature, in the fields of mathematical and experimental nuclear technology, including fission, fusion (blanket physics, radiation damage), safety, materials aspects, economics, etc.
3) Review papers, which may occasionally be invited, are particularly sought by the journal in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


