马来酸酐衍生物查尔酮的合成、表征、抗焦虑和抗惊厥活性、DFT、分子对接和 DMPK 研究
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
焦虑通常不会导致抽搐,但强烈的压力会降低易感人群的抽搐阈值。众所周知,查耳酮可直接作用于中枢神经系统(CNS),而芳香环上不同位置的电子供体和受体基团可改变分子的性质。因此,这项工作研究了新查尔酮衍生物 (E)-6-(4-((E)-3-(3- nitrophenyl)acryloyl)phenyl)-5-oxohex-2-enoic acid (CAMEL) 的抗焦虑和抗惊厥潜力。1H 和 13C NMR 以及 ATR-FTIR 分析有助于确定这种查尔酮的分子结构。能隙分析和高于软度的硬度值证实了 CAMEL 的稳定性,而 CGDRs 则表明它具有更强的亲电性。关于其潜在的抗焦虑作用,测试剂量为 40 毫克/千克的该衍生物表现出与地西泮类似的行为,通过 GABAA 发挥活性。此外,在 20 毫克/公斤-1 的剂量下,该衍生物还显示出可能的抗惊厥作用,与地西泮一样,显示出 GABAA 受体在这一过程中的第 2 和第 3 阶段的参与。鉴于 CAMEL 在体内和硅学测试中表现出的抗焦虑和抗惊厥活性,它有望成为治疗影响中枢神经系统疾病的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
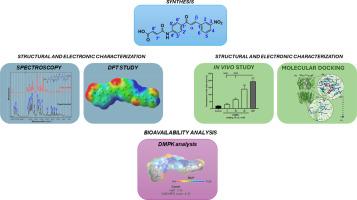
Synthesis, characterization, anxiolytic and anticonvulsant activity, DFT, molecular docking, DMPK studies of chalcone derived from maleic anhydride
Anxiety typically doesn't cause convulsions, but intense stress can reduce the threshold for convulsive bouts in predisposed individuals. Chalcones are known to act directly on the central nervous system (CNS) and the presence of electron donor and acceptor groups attached to the aromatic rings in various positions can alter the properties of the molecule. Thus, this work investigated the anxiolytic and anticonvulsant potential of the new chalcone derivative (E)-6-(4-((E)-3-(3-nitrophenyl)acryloyl)phenyl)-5-oxohex-2-enoic acid (CAMEL). 1H and 13C NMR and ATR-FTIR analyses helped to determine the molecular structure of this chalcone. The energy gap analysis and the higher hardness values than the softness values confirm the stability of CAMEL, with CGDRs indicating that it is more electrophilic in nature. In relation to its potential anxiolytic effect, the tested dose of 40 mg kg-1 of the derivative showed behavior like Diazepam, with activity via GABAA. In addition, the derivative also exhibited a possible anticonvulsant effect at the dose of 20 mg kg-1, being like Diazepam, showing involvement in stages 2 and 3 of GABAA receptors in this process. Given the anxiolytic and anticonvulsant activity shown in vivo and in silico tests, CAMEL is a promising candidate for the treatment of diseases that affect the CNS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


