二苯并氧蒽-β-内酰胺混合物作为潜在的抗氧化剂和抗癌剂:合成、生物学评价和对接研究
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在本研究中,我们通过[2 + 2]环加成法设计并合成了一系列在β-内酰胺环的 N-1 位上连接有 14-苯基-14H-二苯并[a,j]呫吨的 β-内酰胺化合物。所有合成化合物都通过元素分析、傅立叶变换红外光谱(FT-IR)和核磁共振(1H NMR 和 13C NMR)光谱分析进行了表征。对这些衍生物的抗癌和抗氧化活性进行了评估。通过 3-(4,5-二甲基噻唑基-2)-2,5-二苯基溴化四氮唑(MTT)试验,考察了新化合物对 MCF-7(乳腺癌)、HepG2(肝癌)和 TC-1(小鼠肺上皮细胞)细胞系的体外抗癌活性。值得注意的是,化合物 4c、4e 和 4 g 对 TC-1 细胞株具有显著的抗癌活性。此外,化合物 4c、4f 和 4 g 对 MCF-7 细胞株也有很好的疗效。二苯基吡啶肼(DPPH)自由基清除试验表明,4 h 和 4i 是比维生素 C 更有效的抗氧化剂,而 4a、4b、4c 和 4j 则表现出中等程度的抗氧化能力。此外,对接模拟结果与生物检测结果一致,表明这些混合物可能通过氢键和其他非价相互作用与蛋白质靶标结合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
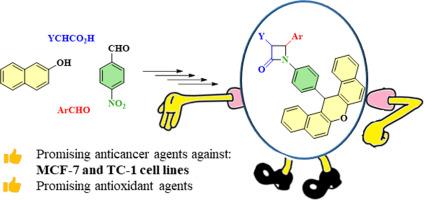
Dibenzoxanthene-β-lactam hybrids as potential antioxidant and anticancer agents: Synthesis, biological evaluation, and docking study
In this study, we designed and synthesized a series of β-lactam compounds bearing 14-phenyl-14H-dibenzo[a,j]xanthene moiety attached to the N-1 position of the β-lactam ring through a [2 + 2] cycloaddition process. All the synthesized compounds have been characterized by elemental analysis, Fourier-transform infrared (FT-IR) and nuclear magnetic resonance (1H NMR and 13C NMR) spectral analysis. These derivatives were evaluated for their anticancer and antioxidant activities. The in vitro anticancer activity of new compounds was examined against MCF-7 (breast cancer), HepG2 (liver hepatocellular carcinoma), and TC-1 (mouse lung epithelial) cell lines by 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay. Notably, compounds 4c, 4e and 4 g demonstrated significant anticancer activity against TC-1 cell line. Additionally, compounds 4c, 4f and 4 g exhibited promising levels of efficacy against the MCF-7 cell line. The diphenylpicrylhydrazyl (DPPH) radical scavenging assay indicated that 4 h and 4i are more potent antioxidant agents than the Vitamin C, while 4a, 4b, 4c, and 4j exhibited moderate antioxidant capabilities. Furthermore, the docking simulations are consistent with the biological assay results, indicating that the hybrids may bind to protein targets through hydrogen bonding and other nonvalent interactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


