通过抑制 PI3K/AKT 通路提高抗肿瘤活性的 H2S 释放型奥利多宁衍生物
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
通过释放硫化氢(H2S)激活细胞程序性死亡已成为一种前景广阔的肿瘤治疗策略。Oridonin 因其独特的支架和广泛的生物效应,尤其是抗肿瘤特性,成为药物开发的先导化合物。在前期结构-活性关系研究的基础上,合成了 33 个新型 1-O/14-O 释放 H2S 的奥利多宁衍生物。其中,11a 的抗增殖活性最强,能有效抑制 MCF-7 和 MIA-PaCa-2 细胞的集落形成、迁移和侵袭。它还能抑制 PI3K/AKT 通路,调节 Bax 和 Bcl-2 的表达,从而启动 Caspase 级联反应,激活线粒体介导的细胞凋亡。此外,11a 还能抑制乳腺癌合成模型中的肿瘤生长,且无明显毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
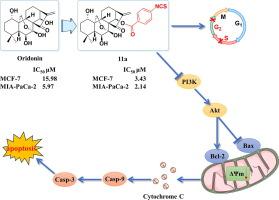
H2S-releasing oridonin derivatives with improved antitumor activity by inhibiting the PI3K/AKT pathway
Activating programmed cell death by delivering hydrogen sulfide (H2S) has emerged as a promising strategy for tumor therapy. Oridonin serves as a lead compound for drug development due to its unique scaffold and wide-ranging biological effects, especially its antitumor properties. Based on the previous structure–activity relationship studies, 33 novel 1-O/14-O H2S-releasing oridonin derivatives were synthesized. Particularly, 11a exhibited the most potent antiproliferative activity, effectively inhibiting colony formation, migration and invasion in both MCF-7 and MIA-PaCa-2 cells. It also inhibited the PI3K/AKT pathway to regulate the expression of Bax and Bcl-2, thereby initiating the Caspase cascade to activate mitochondrial mediated apoptosis. Furthermore, 11a suppressed tumor growth in breast cancer syngeneic models with no apparent toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


