从柠檬酸铋中提取的 Bi3O4Br 光催化剂对甲基溴的降解效率出乎意料地高
IF 3.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
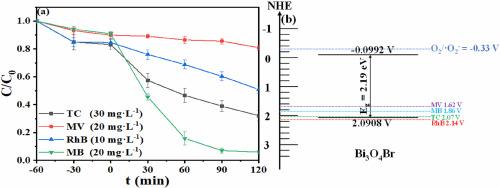
以柠檬酸铋为铋源,不加任何表面活性剂,在 180 °C、pH 值为 12.5 的条件下,采用水热法首次合成了具有均匀方片状结构的纯 Bi3O4Br。结果表明,所制备的 Bi3O4Br 对亚甲蓝(MB)、四环素(TC)、甲基紫(MV)和罗丹明 B(RhB)具有优异的光降解效率,其表观速率常数(ka)为 0.02676 min-1,分别优于 TC、MV 和 RhB(ka = 0.00899 min-1、0.00073 min-1 和 0.00409 min-1)。由于空穴的直接氧化在降解过程中占主导地位,对光催化机理的研究表明,Bi3O4Br 的价带(VB)电位比 MB 的 HOMO 轨道位置更正是先决条件,而且光催化剂的 VB 电位与污染物的 HOMO 能级之间的差值越小,空穴迁移和随后的污染物氧化速度就越快。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unexpectedly high degradation efficiency for MB on Bi3O4Br photocatalyst obtained from bismuth citrate
For the first time, pure Bi3O4Br with a uniform square sheet-like structure was synthesized using a hydrothermal method at 180 °C and pH of 12.5 by employing bismuth citrate as the bismuth source without any surfactant. The photocatalytic activity of Bi3O4Br for the degradation of methylene blue (MB), tetracycline (TC), methyl violet (MV), and rhodamine B (RhB) was evaluated under visible light illumination and the results showed that the as-prepared Bi3O4Br exhibited excellent photodegradation efficiency for MB with an apparent rate constant (ka) of 0.02676 min−1, superior to TC, MV and RhB with ka = 0.00899 min−1, 0.00073 min−1 and 0.00409 min−1, respectively. Since the direct oxidation of the holes predominates in the degradation process, the study on the photocatalytic mechanism suggested that a more positive valence band (VB) potential of Bi3O4Br than HOMO orbital position of MB was prerequisite and also, the smaller the difference between VB potential of a photocatalyst and HOMO energy level of a pollutant, the faster the migration of the holes and subsequent oxidation of pollutant.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


