大环硫醇锌胺的实用合成
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of the American Chemical Society
Pub Date : 2024-10-12
DOI:10.1021/jacs.4c1127010.1021/jacs.4c11270
引用次数: 0
摘要
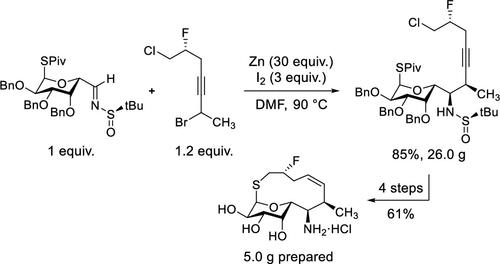
本文介绍了两种结构复杂的候选抗生素 BT-33 和链霉素的北部大环硫醇锌胺片段的可扩展合成方法。每种途径中的一个关键转化是使用锌促进的巴比尔型丙炔化协议,将一个推定的烯锌亲核体与常见的埃尔曼亚磺酰亚胺中间体进行高非对映选择性加成,本文将对此进行详细介绍。这些转化过程具有动态动力学分辨率,且每种丙炔基溴化物前体的用量仅为 1.2 等量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Practical Synthesis of Macrobicyclic Thiolincosamines
Scalable syntheses of the northern macrobicyclic thiolincosamine fragments of two structurally complex antibiotic candidates, BT-33 and cresomycin, are presented. A key transformation in each route is the highly diastereoselective addition of a putative allenylzinc nucleophile to a common Ellman sulfinimine intermediate using a zinc-promoted Barbier-type propargylation protocol that is detailed herein. These transformations proceed with dynamic kinetic resolution and use just 1.2 equiv of each respective propargyl bromide precursor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


