FUT3 通过合成 ITGA6 和 GLG1 上的 Lea 来促进胃癌细胞的迁移,从而影响粘附性和囊泡分布。
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
目的:Lewis抗原在胃癌(GC)进展过程中起着重要作用,FUT3是合成Lewis抗原的关键酶,但其促进GC进展的分子机制尚不清楚:主要方法:利用Lea-抗体捕获结合质谱鉴定FUT3的靶蛋白,并进行免疫荧光(IF)、分子生物学和细胞功能实验,阐明FUT3通过调控ITGA6和GLG1上的Lea糖基化促进GC细胞迁移和侵袭的分子机制:FUT3促进GC细胞的迁移和侵袭。FUT3在GC细胞中的沉默导致整合素α6β4在质膜上聚集,与病灶粘附和半膜小体相关,并减少了GLG1在细胞囊泡中的分布。IGP分析显示,ITGA6的2个聚糖的10个N-聚糖和GLG1的4个聚糖的31个N-聚糖中存在Lea结构。沉默 ITGA6 会促进迁移和侵袭,而沉默 GLG1 则会抑制 GC 细胞的这些过程,这些过程受 FUT3 介导的 Lea 合成的调节:总之,FUT3促进GC细胞迁移和侵袭是由于Lea在ITGA6和GLG1上的合成,前者影响细胞粘附,后者影响细胞内囊泡的分布。这些发现可能有助于开发新的治疗靶点,以抑制或控制 GC 细胞的转移行为,并加深我们对 Lea 在调节蛋白质功能方面作用的了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
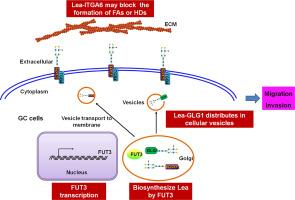
FUT3 promotes gastric cancer cell migration by synthesizing Lea on ITGA6 and GLG1, affecting adhesion and vesicle distribution
Aims
Lewis antigen plays an important role in the progression of gastric cancer (GC), FUT3 is a key enzyme in the synthesis of Lewis antigen, but the molecular mechanism of its promotion of GC progression remains unclear.
Main methods
We used Lea-antibody capturing coupled with mass spectrometry to identify the target proteins of FUT3, immunofluorescence (IF), molecular biology and cell function experiments were conducted to clarify the molecular mechanism of FUT3 promoting the migration and invasion of GC cells by regulating Lea glycosylation on ITGA6 and GLG1.
Key findings
FUT3 promote migration and invasion of GC cells. FUT3 silencing in GC cells led to the aggregation of integrin α6β4 on the plasma membrane, associated with focal adhesion and hemidesmosome, and decreased GLG1 distribution in cellular vesicles. IGP analysis revealed Lea structure in 10 N-glycans of 2 glycosites for ITGA6 and 31 N-glycans of 4 glycosites for GLG1. Silencing ITGA6 promoted migration and invasion, while silencing GLG1 inhibited these processes in GC cells, regulated by FUT3-mediated Lea synthesis.
Significance
In conclusion, FUT3's promotion of GC cell migration and invasion is attributed to Lea synthesis on ITGA6, impacting cell adhesion, and on GLG1, influencing distribution in intracellular vesicles. These findings may contribute to developing novel therapeutic targets for inhibiting or controlling the metastatic behavior of GC cells and enhancing our understanding of Lea's role in regulating protein functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


