血管钙化的表观遗传调控:探寻 sirt1 和组蛋白乙酰化在血管内皮细胞表型转变中的作用。
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
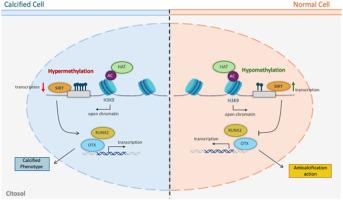
鉴于异位血管钙化的复杂起源及其对健康的重大影响,本研究对支配血管平滑肌细胞(VSMC)的分子动力学进行了全面探索。我们以表观遗传调控为重点,研究了血管平滑肌细胞从收缩表型向钙化表型的转变,并着重了解了 SIRT1 的作用。为此,我们使用了单批人类主动脉SMCs,在规定的通过数下使用以保持一致性,并对其进行长达72小时的钙和磷酸盐超载。我们的研究结果经 RT q-PCR、Western 印迹、免疫荧光和 DNA 甲基化分析验证,揭示了在这种表型转变过程中乙酰转移酶和去乙酰化酶之间复杂的相互作用。我们强调了 HAT1A 在组蛋白乙酰化调控中的关键作用以及 HDAC 的参与,亚细胞定位研究也证明了这一点。此外,我们还证明了第三类去乙酰化酶 SIRT1 的表达在血管内皮细胞钙化过程中的调节作用,强调了 DNA 甲基化在这一过程中的影响。重要的是,该研究探讨了以前未曾探索过的动态蛋白质表达模式,为 Runx2 和 osterix 等关键蛋白质的反直觉表达提供了见解。这项研究强调了表观遗传机制,特别是 SIRT1 的调控,在血管内皮细胞从收缩表型向钙化表型转变过程中的重要影响,为进一步探索血管钙化提供了潜在的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Epigenetic modulation of vascular calcification: Looking for comprehending the role of sirt1 and histone acetylation in VSMC phenotypic transition
In light of the complex origins of ectopic vascular calcification and its significant health implications, this study offers a comprehensive exploration of the molecular dynamics governing vascular smooth muscle cells (VSMCs). Focusing on epigenetic modulation, we investigate the transition from a contractile to a calcifying phenotype in VSMCs, with an emphasis on understanding the role of SIRT1. For this purpose, a single batch of human aortic SMCs, used at a specified passage number to maintain consistency, was subjected to calcium and phosphate overload for up to 72 h. Our findings, validated through RT q-PCR, Western blot, immunofluorescence, and DNA methylation analyses, reveal a complex interplay between acetyltransferases and deacetylases during this phenotypic transition. We highlight HAT1A's critical role in histone acetylation regulation and the involvement of HDACs, as evidenced by subcellular localization studies. Moreover, we demonstrate the modulation of SIRT1 expression, a class III deacetylase, during VSMC calcification, underscoring the influence of DNA methylation in this process. Importantly, the study addresses previously unexplored aspects of the dynamic protein expression patterns observed, providing insight into the counterintuitive expressions of key proteins such as Runx2 and osterix. This research underscores the significant impact of epigenetic mechanisms, particularly the modulation of SIRT1, in the transition from a contractile to a calcifying phenotype in VSMCs, offering potential avenues for further exploration in the context of vascular calcification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


