基于人体组织的肾脏炎症模型
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
炎症在各种肾脏疾病的发生和发展过程中起着关键作用。然而,炎症导致不同病因引起的肾脏疾病的具体分子和细胞机制仍有待阐明。为了加深对这些机制的理解,我们需要一个可靠的、可转化的肾脏炎症人体模型。在这里,我们旨在利用人体精密切割肾切片(PCKS)建立这样一个模型。PCKS 取自新鲜、宏观上健康的肾脏组织,用或不用肿瘤坏死因子 α(TNFα)或其抑制剂 Etanercept 培养 3-48 小时。随后使用 qPCR 和细胞因子阵列对切片中的炎症反应进行了评估。此外,还使用免疫荧光染色法观察了免疫细胞的存在,并用酶联免疫吸附试验检测了组织驻留巨噬细胞的活化潜能。我们观察到培养诱导的炎症反应,这反映在促炎症基因 TNF、IL1B、CCL2 和 IL6 的表达增加。Etanercept 可以部分抑制这种反应,这表明 TNFα 在观察到的反应中起了作用。此外,我们还发现 TNFα 的刺激进一步增加了 TNF、IL1B、CCL2 和 IL6 的基因表达,以及几种趋化因子和细胞因子的产生,包括 CXCL5、MCP1、MCP3 和 IL-6。最后,我们观察到培养期间切片中存在 HLA-DR 阳性细胞以及增殖(CD68 和 PCNA 阳性)和活化的巨噬细胞。总之,这项研究为研究肾脏炎症提供了一种新的人体模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
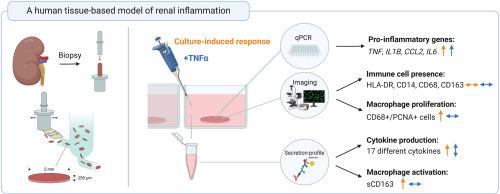
A human tissue-based model of renal inflammation
Inflammation plays a key role in both the onset and progression of various kidney diseases. However, the specific molecular and cellular mechanisms by which inflammation drives kidney diseases from different etiologies remain to be elucidated. To enhance our understanding of these mechanisms, a reliable and translational human model of renal inflammation is needed.
Here, we aim to establish such a model using human precision-cut kidney slices (PCKS). The PCKS were prepared from fresh, macroscopically healthy kidney tissue and cultured for 3h–48h with or without tumor necrosis factor-α (TNFα), or its inhibitor Etanercept. The ensuing inflammatory response in the slices was evaluated using both qPCR and a cytokine array. Furthermore, the presence of immune cells was visualized using immunofluorescent staining, and the activation potential of tissue-resident macrophages was examined with ELISA.
We observed a culture-induced inflammatory response, reflected by increased expression of pro-inflammatory genes TNF, IL1B, CCL2, and IL6. This response could be partially inhibited by Etanercept, indicating that TNFα plays a role in the observed response. Moreover, we found that TNFα stimulation further increased the gene expression of TNF, IL1B, CCL2, and IL6, as well as the production of several chemokines and cytokines, including CXCL5, MCP1, MCP3, and IL-6. Lastly, we observed the presence of CD14- and HLA-DR-positive cells, as well as proliferating (CD68- and PCNA-positive) and activated macrophages in the slices during incubation. In conclusion, this study presents a novel human model for investigating renal inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


