铜绿假单胞菌 PAO1 中一种独特的共表达硫基转移酶扩展了巯丙酸二氧酶的生理作用。
IF 2.3
4区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Proteins and proteomics
Pub Date : 2024-10-28
DOI:10.1016/j.bbapap.2024.141059
引用次数: 0
摘要
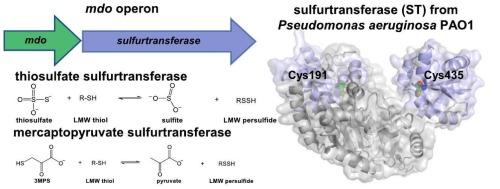
细菌中过硫化物的氧化和同化通常是由过硫化物二氧 化酶和硫转移酶在连续反应中催化的。铜绿假单胞菌 PAO1 中负责氧化过硫化物的酶尚未明确定义。有人提出,铜绿假单胞菌 PAO1 中特征性的巯基丙酸二氧酶(MDO)可催化 3-巯基丙酸的氧化。然而,由于硫醇二氧酶在同一操作子上表达硫转移酶(ST),MDO 的生理作用尚不确定。ST 基因与 mdo 基因的共现频率为 0.94,表明这两个基因的共同表达和生理联系。ST 酶中有四个串联的菱形结构域,其中两个结构域含有能形成过硫化物的潜在催化 Cys 残基(Cys191 和 Cys435)。在硫醇定量分析中,只有 Cys435 是可触及的,H/D-X MS 分析结果进一步确定了含有 Cys435 的结构域的可触及性。硫代硫酸盐和巯基丙酮酸都是 ST 酶的硫供体,Cys435 形成过硫化物中间体。对 MDO 的动力学研究表明,该酶的底物特异性比以前发现的更广,可氧化巯基丙酸盐和巯基丙酮酸盐硫醇和过硫化物底物。这些研究结果有助于深入了解 mdo 操作子在硫化物氧化和同化过程中的总体机制和生理作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A distinct co-expressed sulfurtransferase extends the physiological role of mercaptopropionate dioxygenase in Pseudomonas aeruginosa PAO1
Oxidation and assimilation of persulfides in bacteria is often catalyzed by a persulfide dioxygenase and sulfurtransferase in consecutive reactions. Enzymes responsible for the oxidation of persulfides have not been clearly defined in Pseudomonas aeruginosa PAO1. The characterized mercaptopropionate dioxygenase (MDO) in P. aeruginosa PAO1 has been proposed to catalyze the oxidation of 3-mercaptopropionate. However, the physiological role of MDO is uncertain given the expression of a sulfurtransferase (ST) enzyme on the same operon as the thiol dioxygenase. The st gene had a co-occurrence frequency with mdo of 0.94 demonstrating the co-expression and physiological link of the two genes. There are four tandem rhodanese domains in the ST enzyme with two of the domains containing potential catalytic Cys residues (Cys191 and Cys435) capable of forming a persulfide. Only Cys435 was accessible in thiol quantification assays, and results from H/D-X MS analyses further established the accessibility of the domain containing Cys435. Both thiosulfate and mercaptopyruvate served as sulfur donors to the ST enzyme, with Cys435 forming the persulfide intermediate. Kinetic investigations of MDO suggested the enzyme had a broader substrate specificity than previously identified, oxidizing both mercaptopropionate and mercaptopyruvate thiol and persulfide substrates. The results obtained from these investigations provide insight into the overall mechanism and physiological role of the mdo operon in sulfide oxidation and assimilation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
0.00%
发文量
55
审稿时长
33 days
期刊介绍:
BBA Proteins and Proteomics covers protein structure conformation and dynamics; protein folding; protein-ligand interactions; enzyme mechanisms, models and kinetics; protein physical properties and spectroscopy; and proteomics and bioinformatics analyses of protein structure, protein function, or protein regulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


