4,7,9-三取代苯并氧氮杂卓作为抗利什曼病药物的合成和生物学评价。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
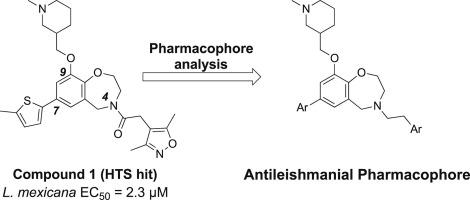
在此,我们报告了一系列抗利什曼病类似物,它们来自 4-[(3,5-二甲基-4-异恶唑基)乙酰基]-9-[(1-甲基-3-哌啶基)甲氧基]-7-(5-甲基-2-噻吩基)-2,3,4,5-四氢-1,4-苯并氧氮杂卓(1),该类似物是通过之前报告的高通量表型筛选确定的。我们设计、合成了该类似物系列,并对其进行了抗利什曼病活性评估,以建立药效元素和初步的结构-活性关系,作为验证该系列的关键步骤,以便进一步优化。这项研究最终确定了早期的先导化合物 46,它对巨噬细胞内的 L. mexicana amastigotes 具有亚微摩级的增殖抑制活性,对宿主巨噬细胞(J774A.1 株)具有适度的选择性,并且具有良好的水溶性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and biological evaluation of 4,7,9-trisubstituted benzoxazepines as antileishmanial agents
Herein we report a series of antileishmanial analogues derived from 4-[(3,5-dimethyl-4-isoxazolyl)acetyl]-9-[(1-methyl-3-piperidinyl)methoxy]-7-(5-methyl-2-thienyl)-2,3,4,5-tetrahydro-1,4-benzoxazepine (1), which was identified through a previously reported high-throughput phenotypic screen. The analogue series was designed, synthesized, and evaluated for antileishmanial activity to establish pharmacophore elements and preliminary structure–activity relationships as key steps in validating the series for further optimization. This study led to identification of the early lead compound 46, which exhibited sub-micromolar proliferation inhibitory activity against intra-macrophage L. mexicana amastigotes, modest selectivity towards host macrophages (J774A.1 line), and good aqueous solubility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


