3-(2-(取代的苄氧基)亚苄基)吡咯烷-2,5-二酮衍生物作为新型 ATX 抑制剂的设计、合成和评估。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
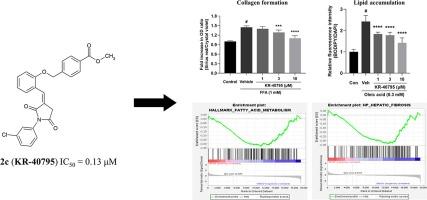
自体表皮生长因子(ATX)已成为治疗肝病的有望靶点。在本研究中,我们通过硅学高通量筛选确定了潜在的候选药物。随后,我们合成了一系列小分子,特别是 KR-40795 (2c),它是一种基于吡咯烷-2,5-二酮的类似物,能与 ATX 的异构隧道和疏水口袋结合。该化合物旨在抑制 ATX 的酶活性,以治疗肝脏疾病。KR-40795 的抑制效力是通过生化试验测定特定底物(FS-3)的水解作用来评估的。值得注意的是,KR-40795 对肝细胞中胶原蛋白的形成和脂质的积累都有明显的抑制作用,这表明它具有治疗肝病(尤其是肝纤维化和脂肪变性)的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis and evaluation of 3-(2-(substituted benzyloxy)benzylidene) pyrrolidine-2,5-dione derivatives for novel ATX inhibitor
Autotaxin (ATX) has emerged as a promising therapeutic target for liver diseases. In this study, we identified potential drug candidates through in silico high-throughput screening. Subsequently, we synthesized a series of small molecules, specifically KR-40795 (2c), a pyrrolidine-2,5-dione-based analogue that binds to the allosteric tunnel and hydrophobic pocket of ATX. This compound was designed to inhibit the enzymatic activity of ATX for the treatment of liver diseases. The inhibitory potency of KR-40795 was evaluated using a biochemical assay that measured the hydrolysis of a specific substrate (FS-3). Notably, KR-40795 demonstrated significant inhibition of both collagen formation and lipid accumulation in liver cells, suggesting its potential as a therapeutic agent for liver diseases, particularly fibrosis and steatosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


