通过联合使用 PARP1-PROTAC 和 BRD4 抑制剂提高同源重组缺陷胰腺癌的疗效。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
目前,美国食品和药物管理局已批准将多(ADP-核糖)聚合酶抑制剂(PARPi)用于治疗 BRCA 基因突变的胰腺癌。然而,有限的适应症阻碍了它们的进一步应用。抑制含溴结构域蛋白 4(BRD4)可阻断同源重组(HR)修复途径,并有可能增强 HR 特异性胰腺癌治疗对 PARPi 的反应。此外,蛋白水解靶向嵌合体(PROTACs)可以劫持细胞内的E3连接酶,有效、快速地泛素化降解靶向蛋白,从而增强对肿瘤的治疗效果。在本研究中,诱导PARP1降解的LB23化合物与BRD4抑制剂JQ1联用,通过多种方法证实了它们在HR缺陷型胰腺癌中的协同作用。此外,与JQ1和PARPi olaparib联用相比,PARP1-PROTAC和JQ1的协同作用更为显著。对协同作用机制的进一步研究表明,联合疗法通过诱导细胞周期停滞和细胞凋亡,增强了DNA损伤,抑制了DNA修复。因此,本研究为这种联合疗法提供了实验数据,有望成为治疗HR阳性胰腺癌的一种创新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
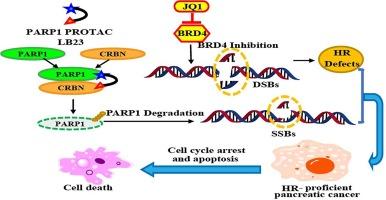
Enhancing therapeutic efficacy in homologous recombination-proficient pancreatic cancer via the combination of PARP1-PROTAC and a BRD4 inhibitor
Currently, poly (ADP-ribose) polymerase inhibitors (PARPi) have been approved by U.S. Food and Drug Administration for BRCA-mutated pancreatic cancer therapy. However, limited indications hinder their further application. Repression of bromodomain-containing protein 4 (BRD4) can block the homologous recombination (HR) repair pathway and has the potential to enhance the response to PARPi in HR-proficient pancreatic cancer therapy. In addition, proteolysis targeting chimeras (PROTACs) can hijack E3 ligase within the cell to ubiquitinate degradation-targeted proteins effectively and quickly, thus enhancing the therapeutic effect on tumors. In the present study, the LB23 compound, which induces PARP1 degradation, was employed in combination with the BRD4 inhibitor JQ1, confirming their synergistic effect in HR-proficient pancreatic cancer through various methods. Moreover, compared to the JQ1 and PARPi olaparib combination, PARP1-PROTAC and JQ1 had more notable synergistic effects. Further research into the synergistic mechanism demonstrated that combination therapy enhanced DNA damage and suppressed DNA repair by inducing cell cycle arrest and cell apoptosis. The present study therefore provides the experimental data for this type of combination therapy, which is expected to be an innovative approach for the treatment of HR-proficient pancreatic cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


