Miguel Fontecha-Barriuso, Natalia Villar-Gomez, Juan Guerrero-Mauvecin, Julio M Martinez-Moreno, Susana Carrasco, Diego Martin-Sanchez, María Rodríguez-Laguna, Manuel J Gómez, María D Sanchez-Niño, Marta Ruiz-Ortega, Alberto Ortiz, Ana B Sanz
下载PDF
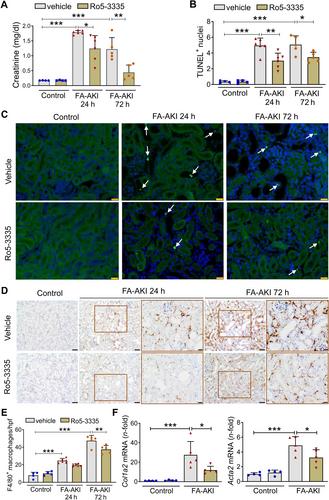
{"title":"Runt 相关转录因子 1 (RUNX1) 是急性肾损伤的介质。","authors":"Miguel Fontecha-Barriuso, Natalia Villar-Gomez, Juan Guerrero-Mauvecin, Julio M Martinez-Moreno, Susana Carrasco, Diego Martin-Sanchez, María Rodríguez-Laguna, Manuel J Gómez, María D Sanchez-Niño, Marta Ruiz-Ortega, Alberto Ortiz, Ana B Sanz","doi":"10.1002/path.6355","DOIUrl":null,"url":null,"abstract":"<p>Treatment for acute kidney injury (AKI) is suboptimal. A better understanding of the pathogenesis of AKI may lead to new therapeutic approaches. Kidney transcriptomics of folic acid-induced AKI (FA-AKI) in mice identified <i>Runx1</i> as the most upregulated RUNX family gene. We then examined the expression of RUNX1 in FA-AKI, in bacterial lipopolysaccharide (LPS)-induced cytokine storm-AKI (CS-AKI), and in human AKI. In cultured mouse tubule cells, we explored the expression and role of RUNX1 in response to the cytokine TWEAK or LPS. A chemical inhibitor of RUNX1 (Ro5-3335) was used in animal models of AKI to test its potential as a therapeutic target. RUNX1 overexpression in FA-AKI was validated at the mRNA and protein levels and localized mainly to tubule cell nuclei. CS-AKI also upregulated kidney RUNX1. Increased tubule and interstitial RUNX1 expression were also observed in human AKI. In cultured mouse tubule cells, the pro-inflammatory cytokine TWEAK and LPS increased RUNX1 and IL-6 expression. Mechanistically, RUNX1 bound to the <i>Il6</i> gene promoter and RUNX1 targeting with the chemical inhibitor Ro5-3335, or a specific small interfering RNA (siRNA), prevented the TWEAK- and LPS-induced upregulation of IL6 through a RUNX1/NFκB1 p50 pathway. <i>In vivo</i>, preventive Ro5-3335 improved kidney function and reduced inflammation in FA-AKI and CS-AKI. However, Ro5-3335 administration after the insult only improved kidney function in CS-AKI. Kidney transcriptomics identified inflammatory genes and transcription factor mRNAs such as <i>Yap1</i> and <i>Trp53</i> as key targets of Ro5-3335 in CS-AKI. In conclusion, RUNX1 contributes to AKI by driving the expression of genes involved in inflammation and represents a novel therapeutic target in AKI. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 4","pages":"396-410"},"PeriodicalIF":5.6000,"publicationDate":"2024-10-29","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6355","citationCount":"0","resultStr":"{\"title\":\"Runt-related transcription factor 1 (RUNX1) is a mediator of acute kidney injury\",\"authors\":\"Miguel Fontecha-Barriuso, Natalia Villar-Gomez, Juan Guerrero-Mauvecin, Julio M Martinez-Moreno, Susana Carrasco, Diego Martin-Sanchez, María Rodríguez-Laguna, Manuel J Gómez, María D Sanchez-Niño, Marta Ruiz-Ortega, Alberto Ortiz, Ana B Sanz\",\"doi\":\"10.1002/path.6355\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Treatment for acute kidney injury (AKI) is suboptimal. A better understanding of the pathogenesis of AKI may lead to new therapeutic approaches. Kidney transcriptomics of folic acid-induced AKI (FA-AKI) in mice identified <i>Runx1</i> as the most upregulated RUNX family gene. We then examined the expression of RUNX1 in FA-AKI, in bacterial lipopolysaccharide (LPS)-induced cytokine storm-AKI (CS-AKI), and in human AKI. In cultured mouse tubule cells, we explored the expression and role of RUNX1 in response to the cytokine TWEAK or LPS. A chemical inhibitor of RUNX1 (Ro5-3335) was used in animal models of AKI to test its potential as a therapeutic target. RUNX1 overexpression in FA-AKI was validated at the mRNA and protein levels and localized mainly to tubule cell nuclei. CS-AKI also upregulated kidney RUNX1. Increased tubule and interstitial RUNX1 expression were also observed in human AKI. In cultured mouse tubule cells, the pro-inflammatory cytokine TWEAK and LPS increased RUNX1 and IL-6 expression. Mechanistically, RUNX1 bound to the <i>Il6</i> gene promoter and RUNX1 targeting with the chemical inhibitor Ro5-3335, or a specific small interfering RNA (siRNA), prevented the TWEAK- and LPS-induced upregulation of IL6 through a RUNX1/NFκB1 p50 pathway. <i>In vivo</i>, preventive Ro5-3335 improved kidney function and reduced inflammation in FA-AKI and CS-AKI. However, Ro5-3335 administration after the insult only improved kidney function in CS-AKI. Kidney transcriptomics identified inflammatory genes and transcription factor mRNAs such as <i>Yap1</i> and <i>Trp53</i> as key targets of Ro5-3335 in CS-AKI. In conclusion, RUNX1 contributes to AKI by driving the expression of genes involved in inflammation and represents a novel therapeutic target in AKI. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"264 4\",\"pages\":\"396-410\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-10-29\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6355\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6355\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6355","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


